Application of purslane amide alkaloid in preparing antioxidant and neuroprotective agent
A technology of purslane amide and neuron protection, which is applied in the fields of anti-toxic agents, nervous system diseases, neuromuscular system diseases, etc., and can solve the problems that the antioxidant and neuroprotective activities have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
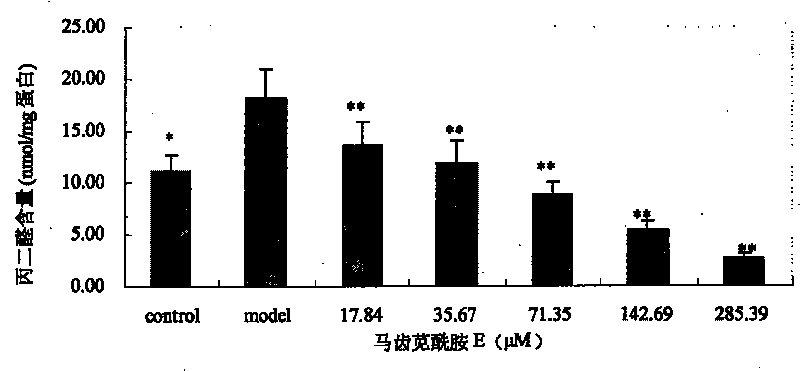
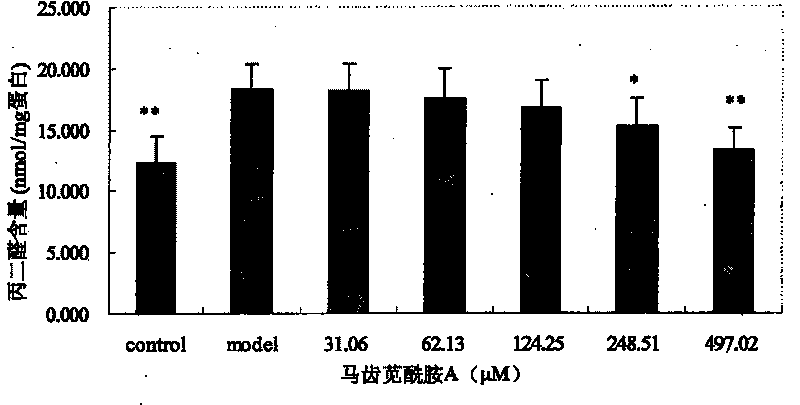
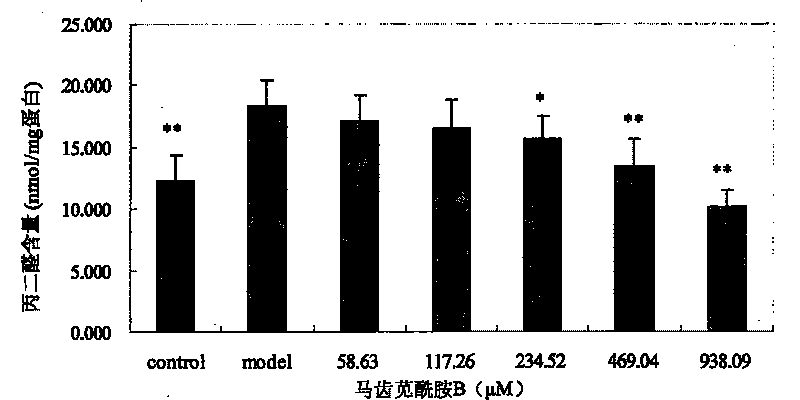
[0040] Embodiment 1: Portulaca oleracea amide alkaloids are used as novel antioxidants
[0041] 1. Experimental Materials and Instruments
[0042] The monomers of purslane amide A, purslane amide B and purslane amide E are extracted and separated from purslane medicinal materials; picryl-hydrazyl, DPPH), vitamin E (α-Tocopherol) and butylated hydroxyanisole (butylated hydroxy anisol, BHA) were purchased from Sigma Company; 1,1,3,3-tetraethoxypropane, thiobaby 2-thiobarbituric acid (TBA) and sodium dodecyl sulfate (SDS) were purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd.; bovine serum albumin and Coomassie brilliant blue G-250 were purchased from Beijing Huamei Transduction Technology Co., Ltd., and other reagents were of analytical grade. The TU-1800 ultraviolet spectrophotometer is produced by Beijing Puxi General Instrument Co., Ltd.; the German-made ULTRA-TURRAX high-shear dispersing emulsifier (homogenizer). Male Wistar rats were provided by the Experiment...
Embodiment 2
[0053] Embodiment 2: Portulaca oleracea amide alkaloids are used as novel neuron protective agents
[0054] 1. Experimental Materials and Instruments
[0055] MEM medium and F12 medium (Gibco), calf serum and fetal bovine serum were purchased from Hangzhou Sijiqing; polylysine and MTT were Sigma products; D-glucose was imported from Sigma; LDH kits were purchased from Beijing Zhongsheng Biological High-Tech Co., Ltd., and the rest of the reagents were domestic analytical grade. Cell culture plates and dishes are Costar products. SANYO (MCO-15AC) carbon dioxide incubator, Olympus inverted microscope and fluorescence microscope; SORVALL refrigerated high-speed centrifuge, T52 ultraviolet-visible spectrophotometer (OHAUS), Bio-RAD microplate reader.
[0056] 2. Experimental steps
[0057] (1) Neuronal cell culture
[0058] Newborn mice (0-3 days) were suffocated to death, sterilized with 75% alcohol for 5 minutes, placed in an ice bath in an ultra-clean bench to separate the ...
Embodiment 3
[0068] Example 3: Portulaca oleracea amide alkaloids are used as ion channel antagonists in neuroprotection
[0069] 1. Experimental Materials and Instruments
[0070] MEM medium and F12 medium (Gibco), calf serum and fetal bovine serum were purchased from Hangzhou Sijiqing; PI, EB, polylysine, Coomassie brilliant blue G250 were Sigma products; D-glucose was imported from Sigma Product; ATPase kit (Nanjing Jiancheng Institute of Bioengineering), and the rest of the reagents were of domestic analytical grade. Cell culture plates and dishes are Costar products. SANYO (MCO-15AC) carbon dioxide incubator, Olympus inverted microscope and fluorescence microscope; SORVALL refrigerated high-speed centrifuge, ZS semi-automatic biochemical analyzer, T52 ultraviolet-visible spectrophotometer (OHAUS), Bio-RAD microplate reader.
[0071] 2. Experimental steps
[0072] The effect of drugs on the ATPase activity on the cell membrane of neurons damaged by hypoxia and glucose: the neurons w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| greyscale | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com