Method for preparing annular aliphatic carbonic ester containing cinnamoyloxy group and polymers thereof
A technology of cinnamoyloxymethyltrimethylene carbonate and cinnamoyloxy, which is applied in the field of preparation of cycloaliphatic carbonate and its polymers, can solve the problems of polylactic acid and its copolymers that have not been seen yet, Achieve good biocompatibility and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
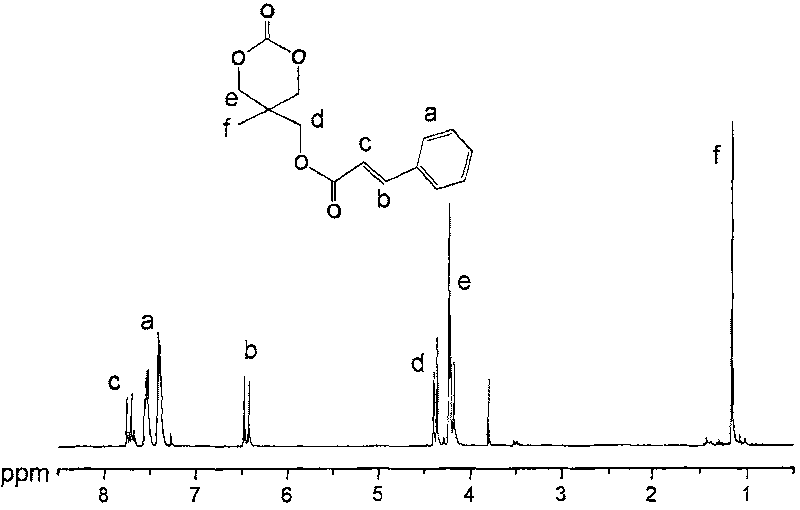
[0050] Preparation of 2-methyl-2-cinnamoyloxymethyltrimethylene carbonate
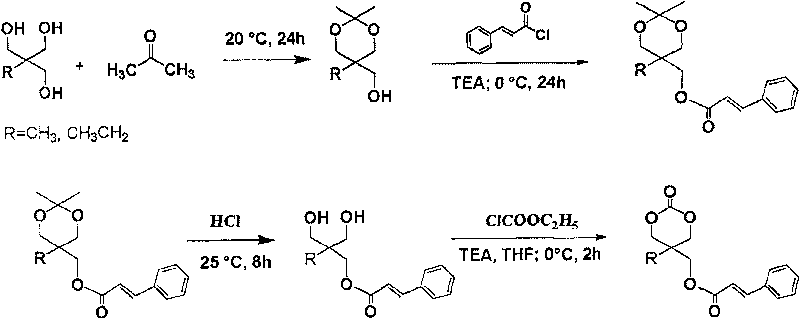
[0051] Dissolve 200g of 2,2-dimethylolpropanol in 2L of acetone, then add 0.2g of p-toluenesulfonic acid, stir the above mixture at room temperature for 2d, then add 5g of anhydrous potassium carbonate, stir for 1h and filter, the filtrate is concentrated, evaporated Remove acetone to obtain 256g 2,2-dimethyl-5-methyl-5-hydroxymethyl-1,3-dioxane; take 20g 2,2-dimethyl-5-methyl-5- Dissolve hydroxymethyl-1,3-dioxane and cinnamoyl chloride in 250ml of tetrahydrofuran, slowly add 30ml of triethylamine dropwise under ice-water bath conditions, react at 0°C for 2 hours, then stir at room temperature for 12 hours, filter out the formed The precipitate, the filtrate was concentrated to no solvent, then added 100ml of methanol and 15ml of concentrated hydrochloric acid, stirred at room temperature for 5h, added 150ml of saturated aqueous sodium carbonate solution, extracted three times with ethyl acetate, each ...
Embodiment 2
[0053] Preparation of 2-ethyl-2-cinnamoyloxymethyltrimethylene carbonate
[0054] Dissolve 67g of 2,2-dimethylol butanol in 400ml of acetone, then add 0.5g of p-toluenesulfonic acid, stir the above mixture at room temperature for 20h, then add 1g of anhydrous potassium carbonate, stir for 1h and filter, the filtrate is concentrated, Acetone was evaporated to obtain 68g 2,2-dimethyl-5-ethyl-5-hydroxymethyl-1,3-dioxane; 21g 2,2-dimethyl-5-ethyl-5 -Hydroxymethyl-1,3-dioxane and 15g of cinnamoyl chloride were dissolved in 300ml of tetrahydrofuran, and 30ml of triethylamine was slowly added dropwise in an ice-water bath, reacted at 0°C for 2 hours, then stirred at room temperature for 12 hours, and filtered The generated precipitate was removed, the filtrate was concentrated to no solvent, then 100ml of methanol and 12ml of concentrated hydrochloric acid were added, and after stirring at room temperature for 5h, 150ml of saturated aqueous sodium carbonate solution was added, and ex...
Embodiment 3
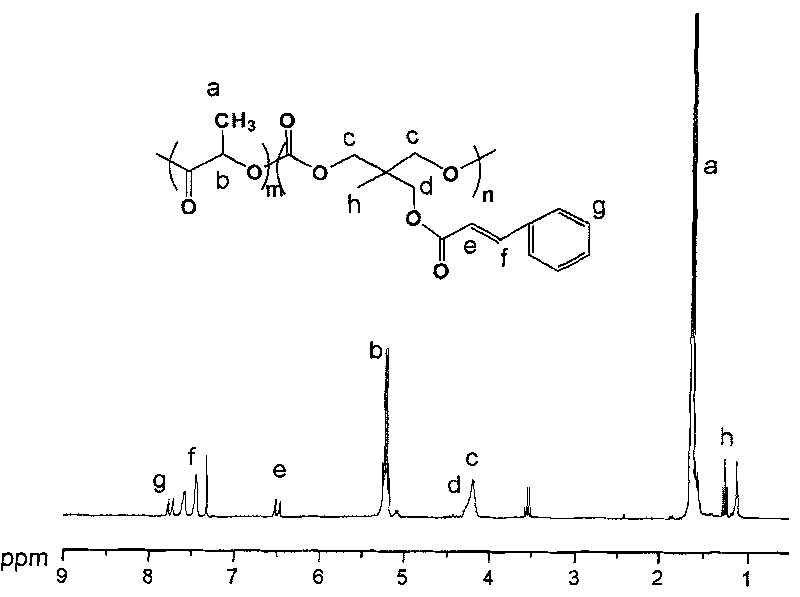
[0056] Preparation of carbonate homopolymer
[0057] Under anhydrous and oxygen-free conditions, 1mol 2-methyl-2-cinnamoyloxymethyl trimethylene carbonate was added to the polymerization reaction bottle, and then ethyl zinc of 1 / 200 of the total amount of monomers was added as The initiator was reacted at 110°C for 15 hours, the product was dissolved in chloroform, settled in methanol, filtered, washed, and vacuum-dried at 35°C to constant weight to obtain a carbonate homopolymer.
[0058] Using 2-ethyl-2-cinnamoyloxymethyl trimethylene carbonate as a monomer, the reaction process and conditions are the same as above to obtain a homopolymer of the monomer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com