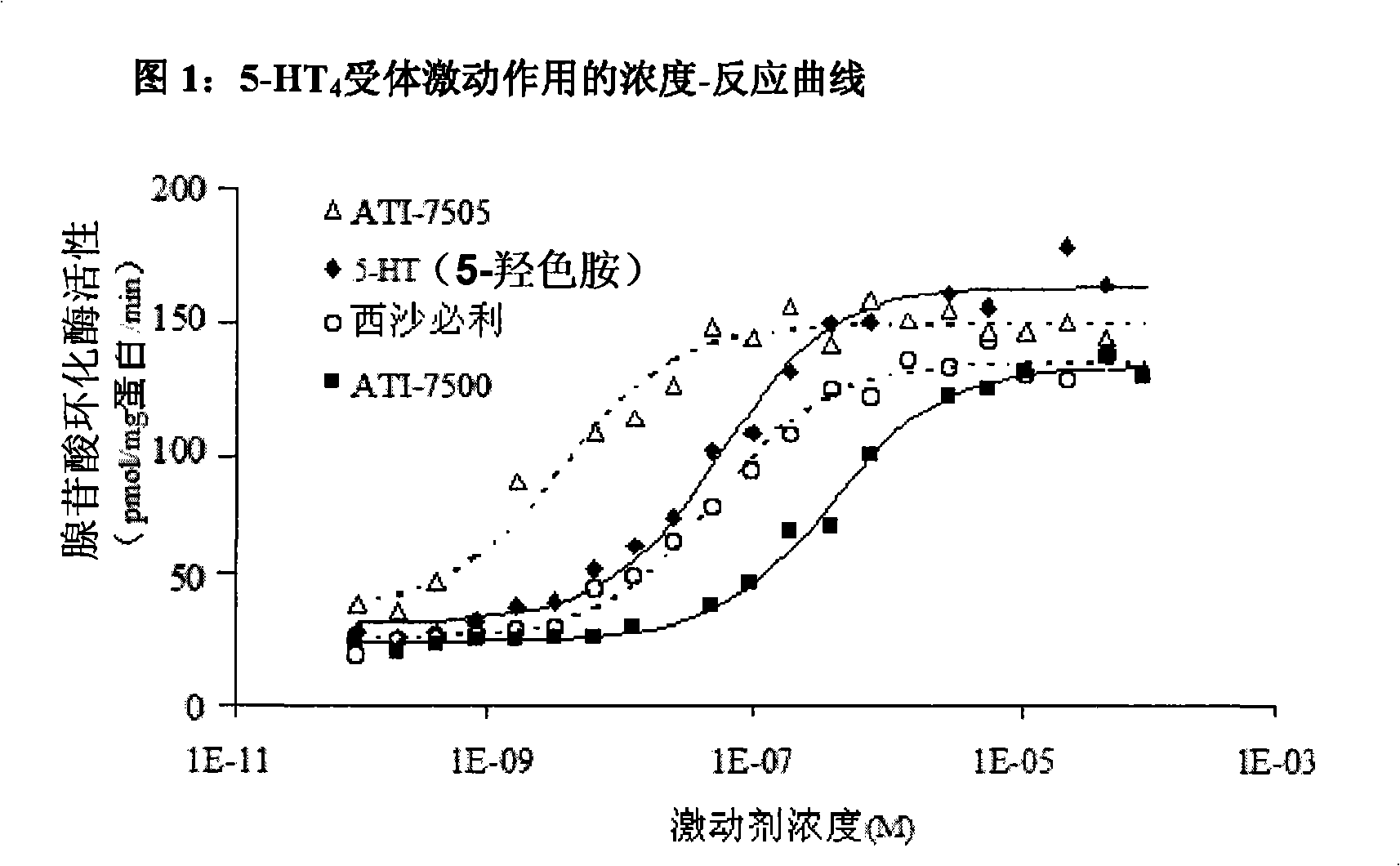
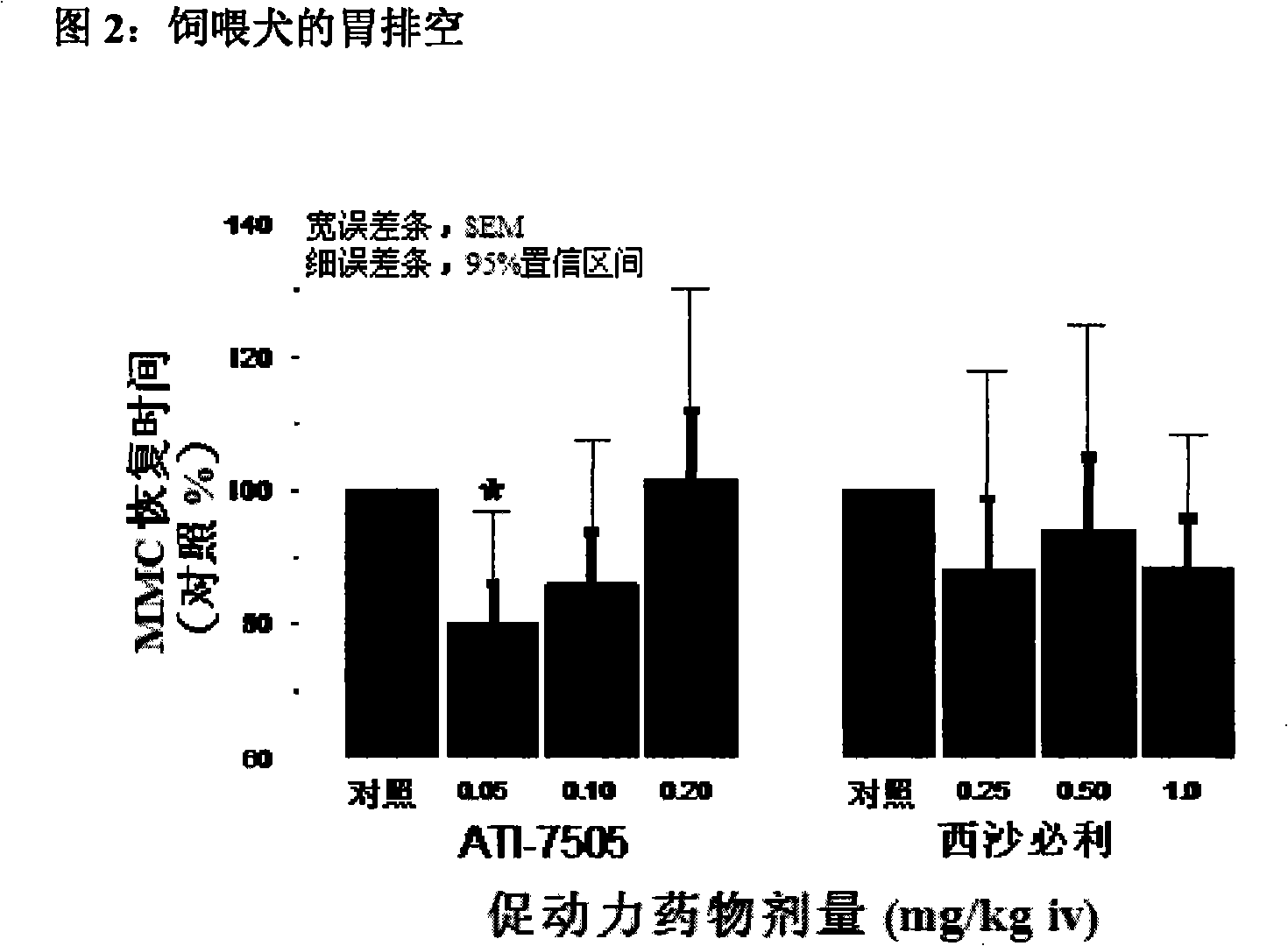
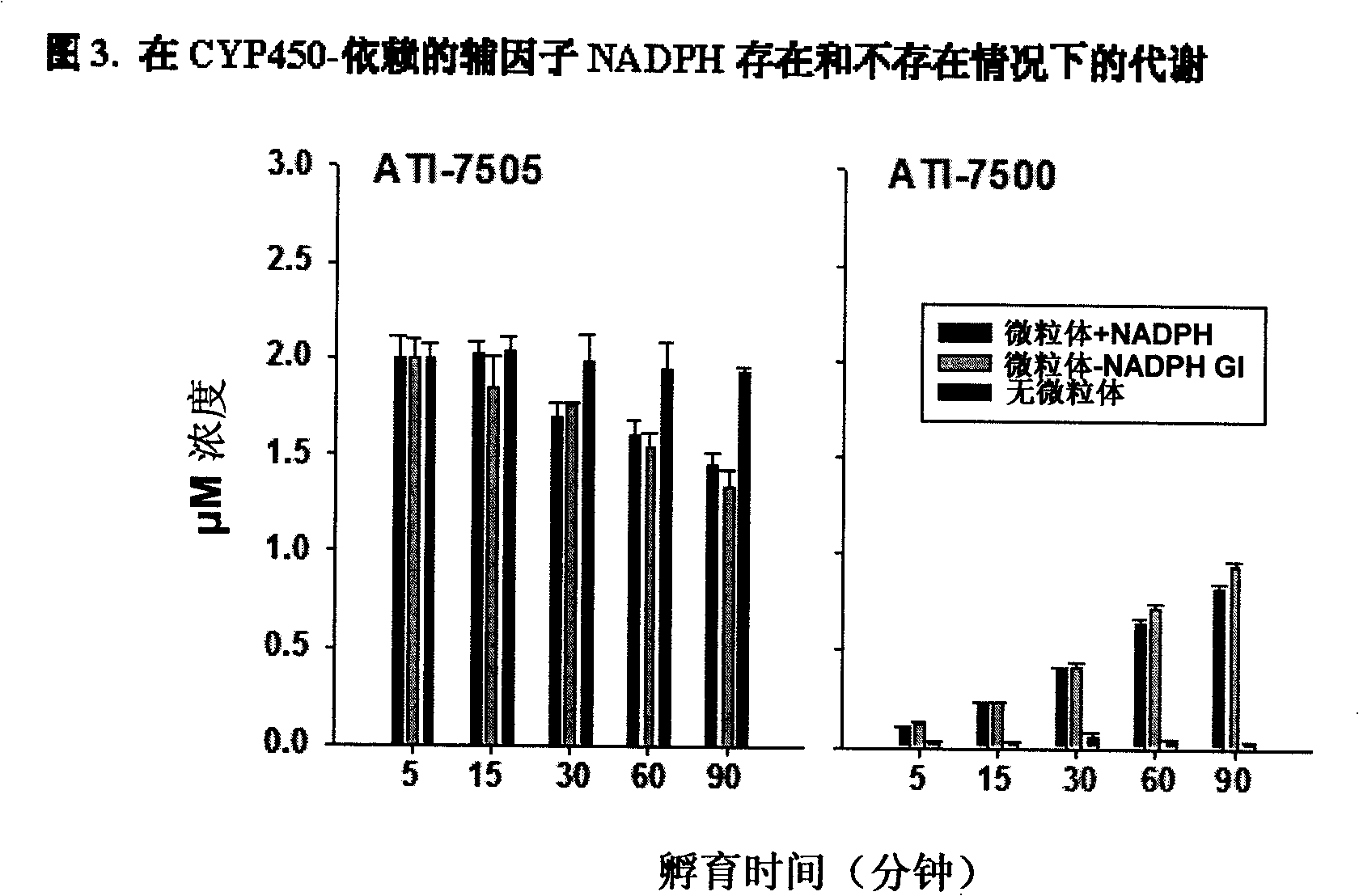
Synthetic methods and intermediates for stereoisomeric compounds useful for the treatment of gastrointestinal and central nervous system disorders
A compound and product technology, which is applied in the field of synthesis and intermediates of stereoisomeric compounds for the treatment of gastrointestinal system and central nervous system diseases, can solve the problems of adverse drug interactions and restrictions, and achieve the occurrence of side effects and toxicity The effect of low rate, reduction of adverse reactions, safe drug metabolism process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0306] Compound preparation
[0307] Although the chemical synthesis of various analogs of cisapride can be carried out according to European Patent Application No. 0,076,530 A2 published on April 13, 1983, WO 01 / 093849, U.S. Patent No. 4,962,115, No. 5,057,525 and Van Daele et al., Drug Development Res. 8:225-232 (1986) (the content of which is incorporated herein by reference in its entirety), the compounds of the present invention are preferably prepared according to the disclosure of Methods 3-6.
Embodiment 1
[0485] 6-[4R-(4-Amino-5-chloro-2-methoxy-benzamido)-3S-methoxy-piperidin-1-yl]-hexanoic acid 1-aza-bicyclo[ 2.2.2] Preparation of octa-3'R-yl ester dihydrochloride
[0486]
[0487] Step 1: Resolution of racemic norcisapride
[0488] (-)-2,3-Dibenzoyl-L-tartaric acid ((-)-DBT, about 1 part by weight) was dissolved in ethanol and filtered to remove residual particles. Separately, racemic norcisapride (approximately 0.8 parts by weight) was dissolved in a mixture of ethanol and water and filtered. The filtrate was heated to about 75°C, and then the (-)-DBT solution was added. After stirring at this temperature for about 30 minutes, the mixture was cooled slowly to about 5 °C for several hours, and the product salt was collected by vacuum filtration and washed with EtOH / H 2 O mixture wash. The filter cake was removed from EtOH / H by heating to about 79°C and then slowly cooling to about 2 recrystallized from O. The product was collected on a vacuum filter and washed with ...
example 2
[0497] According to U.S. Patent No. 6,147,093, or "Enantiomaers, Racemates and Resolutions" by J. Jacques, A. Collet and S.H. Wilen (Wiley-Interscience, New York, NY) (Enantiomers, Racemates and Resolutions) separation), or by the method described in S.H.Wilen et al., Tetrahedron (1977) 33:2725, by resolution of enantiomers using conventional means such as optical resolution acids, can be prepared from their racemic mixture ( +) and (-)-norcisapride.
[0498] The 4 isomers were obtained in low amounts (mg) by preparative column chromatography followed by evaporation of the solvent. This method can be used to prepare small amounts of isomers for analysis and identification purposes. This is a standard separation method routinely used by analytical laboratories to isolate and identify metabolites.
[0499] The possible routes for the synthesis of compound IV, compound VI and (+)-compound II using (+)-norcisapride as starting materials are described below. Except for using (-)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com