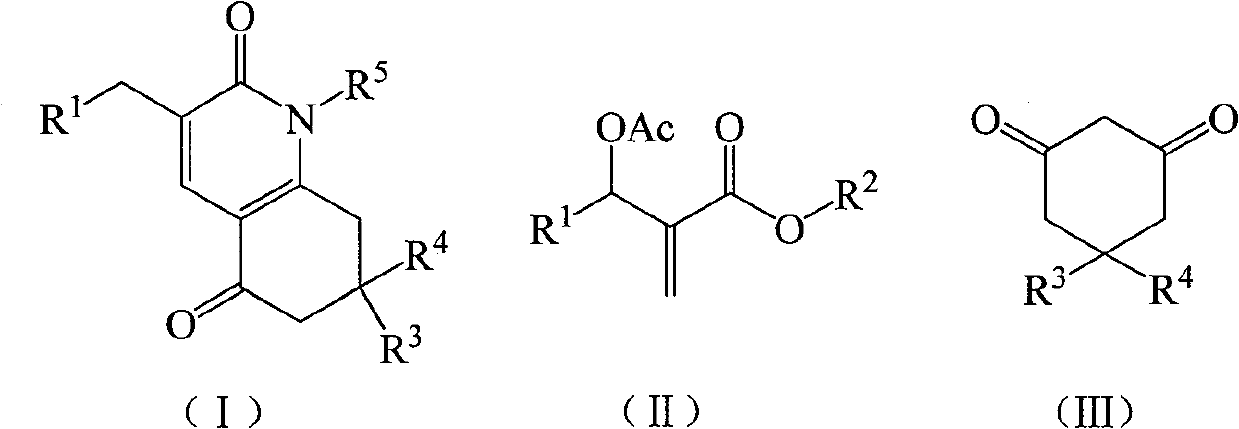
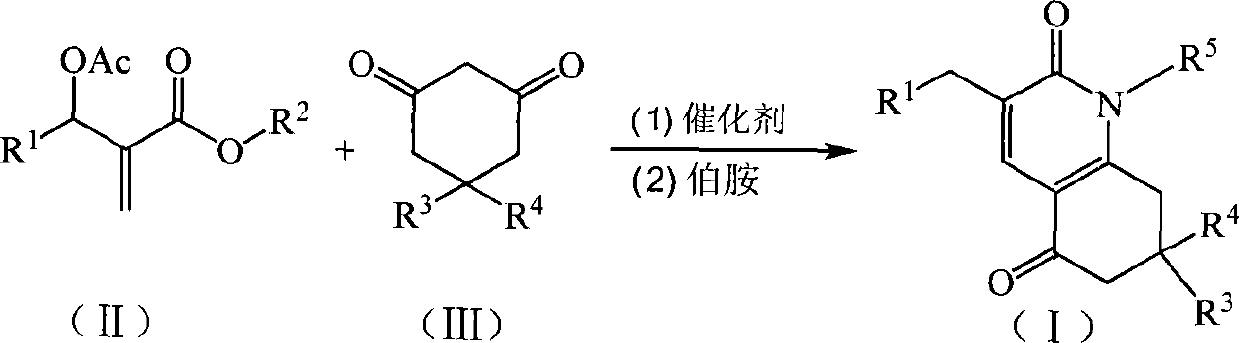
A kind of preparation method of 7,8-dihydroquinoline-2,5(1h,6h)-dione derivatives
A technology for dihydroquinoline and derivatives, applied in 7 fields, can solve the problems of expensive catalyst, harsh reaction conditions, low reaction yield, etc., and achieve the effects of large implementation value, easy availability of raw materials, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 1-phenyl-7,7-dimethyl-3-(3-nitrophenyl)-7,8-dihydroquinoline-2,5(1H,6H)-dione (compound a ) preparation
[0026] In a 250mL three-necked flask equipped with a thermometer, a reflux condenser, and mechanical stirring, add 11.16g (40mmol) of 2-(acetoxy-(3-nitrophenyl))methyl methacrylate, 5,5-di Methyl-1,3-cyclohexanedione 5.60g (40mmol), potassium carbonate 2.76g (20mmol), acetone 33.48g, stirred at 56°C for 4h, then added aniline 3.72g (40mmol), continued the reaction for 2h. The solvent was recovered under reduced pressure, and the resulting crude product was recrystallized from 95% ethanol to obtain 7.20 g of 1-phenyl-7,7-dimethyl-3-(3-nitrophenyl)-7,8-dihydroquinoline- 2,5(1H,6H)-diketone, off-white crystal, the yield is 44.8%, the melting point is 175.0-177.3°C, and the HPLC purity is 98.6%. 1 H NMR (500MHz, CDCl 3 ): δ=1.01(s, 6H, CH 3 ), 2.29 (s, 2H, CH 2 ), 2.38(s, 2H, CH 2 ), 3.94 (s, 2H, CH 2 ), 7.16(t, J=7.5Hz, 2H, ArH), 7.44(t, J=8.0Hz, 1H, A...
Embodiment 2
[0027] Example 2 1-phenyl-7,7-dimethyl-3-(3-nitrophenyl)-7,8-dihydroquinoline-2,5(1H,6H)-dione (compound a ) preparation
[0028]The feed ratio is Baylis-Hillman adduct: 5,5-dimethyl-1,3-cyclohexanedione: base catalyst: aniline=1:1:1:2, wherein Baylis-Hillman adduct is 2- (Acetoxy-(3-nitrophenyl))methyl methacrylate 11.16g (40mmol), the base is potassium carbonate, the solvent is acetone, the amount is 4 times the amount of the Baylis-Hillman adduct, at 56 ° C Under the first stage of reaction for 8h, the second stage of reaction for 3h.
[0029] Others are the same as Example 1, and the product is 1-phenyl-7,7-dimethyl-3-(3-nitrophenyl)-7,8-dihydroquinoline-2,5(1H,6H)- 13.20 g of diketone, off-white crystals, the yield is 82.1%, the melting point is 175.6-177.5°C, and the HPLC purity is 98.4%.
Embodiment 3
[0030] Example 3 1-phenyl-7,7-dimethyl-3-(3-nitrophenyl)-7,8-dihydroquinoline-2,5(1H,6H)-dione (compound a ) preparation
[0031] The feed ratio is Baylis-Hillman adduct: 5,5-dimethyl-1,3-cyclohexanedione: base catalyst: aniline=1: 1.2: 1.2: 3, wherein Baylis-Hillman adduct is 2- (Acetoxy group-(3-nitrophenyl)) methyl methacrylate 11.16g (40mmol), base is triethylenediamine, solvent is acetonitrile, consumption is 8 times of Baylis-Hillman adduct quality, in At 80°C, the first stage was reacted for 5 hours, and the second stage was reacted for 2 hours.
[0032] Others are the same as Example 1, and the product is 1-phenyl-7,7-dimethyl-3-(3-nitrophenyl)-7,8-dihydroquinoline-2,5(1H,6H)- Diketone 13.44g, off-white crystal, yield 83.6%, melting point 175.3-177.0°C, HPLC purity 98.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com