Anode active material for lithium ion secondary battery and its making method
A cathode active material, secondary battery technology, applied in electrode manufacturing, battery electrodes, chemical instruments and methods, etc., can solve the problems of high price, specific capacity and cycle life can not meet the requirements, etc., to reduce production costs, good electricity Effects of chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
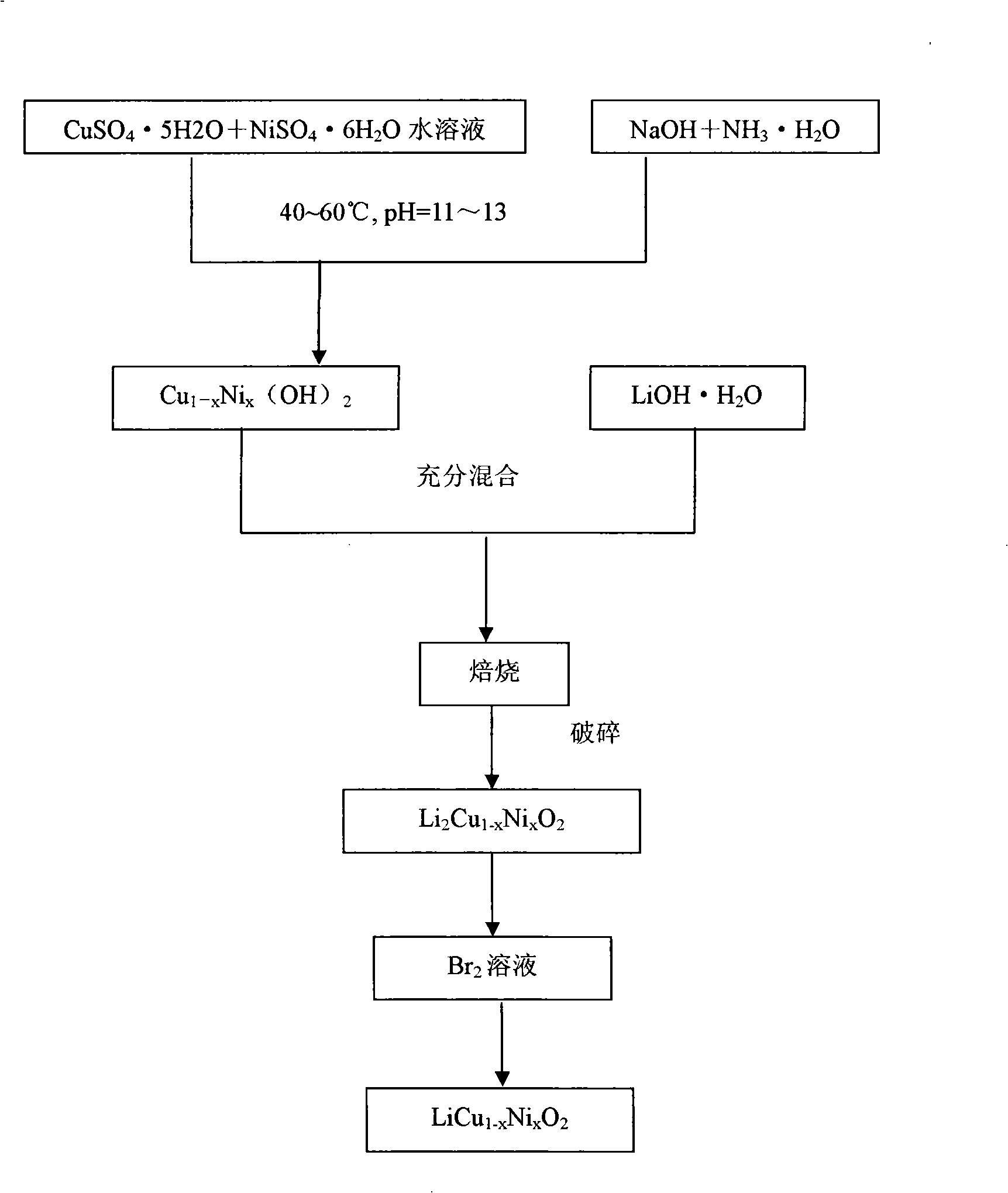
[0020] 10.8kg bile alum (CuSO 4 ·5H 2 O) and 1.8kg nickel sulfate (NiSO 4 ·6H 2 O) dissolved in 25L deionized water to form a mixed solution. In addition, 4.8kg caustic soda (NaOH) and 3L industrial ammonia (NH 3 ·H 2 O) be dissolved in 15L deionized water, and then at 55°C, slowly drop the prepared above-mentioned mixed solution into the reaction vessel under stirring at the same time, and control the pH value to 11.2, and react for 12 hours to obtain the particle size (D 50 ) is 4.5 kg of co-precipitated precursor particles of copper-nickel hydroxide of 15.3 μm.
[0021] The prepared precursor particles were mixed with 0.8kg lithium oxide (Li 2 O) After fully mixing, bake at a temperature of 600°C for 16 hours to obtain copper (II) nickel (II) acid lithium (Li 2 Cu 0.86 Ni 0.14 o 2 )solid. Break the solid into powder and put it into 10L of liquid bromine-acetonitrile solution with a concentration of 2.5% by volume, wash and dry it after oxidation for 5 hours to ob...
Embodiment 2
[0023] 6.2kg bile alum (CuSO 4 ·5H 2 O) and 6.6kg nickel sulfate (NiSO 4 ·6H 2 O) dissolved in 25L deionized water to form a mixed solution. Another 4.8kg caustic soda (NaOH) and 3L ammonia water (NH 3 ·H 2 O) Dissolve in 15 L deionized water. Then at 50°C, the above-mentioned mixed solution prepared was slowly added dropwise to the reaction vessel under stirring at the same time, and the pH value was controlled to be 12.0, and the reaction was carried out for 14 hours to obtain a particle size (D 50 ) is 4.4 kg of co-precipitated precursor particles of copper-nickel hydroxide with a diameter of 17.4 μm.
[0024] The precursor particles were mixed with 0.8kg lithium oxide (Li 2 O) After fully mixing, bake at a temperature of 800°C for 24 hours to obtain copper (II) nickel (II) lithium (Li) 2 Cu 0.86 Ni 0.14 o 2 )solid. Break the solid into powder and put it into 10L of liquid bromine-acetonitrile solution with a concentration of 4.0% by volume, wash and dry it afte...
Embodiment 3
[0026] 10kg bile alum (CuSO 4 ·5H 2 O) and 15.8kg nickel sulfate (NiSO 4 ·6H 2 O) dissolved in 25L deionized water to form a mixed solution. In addition, 4.8kg caustic soda (NaOH) and 3L industrial ammonia (NH 3 ·H 2 O) be dissolved in 15L deionized water, and then at 52°C, slowly drop the prepared above-mentioned mixed solution into the reaction vessel under stirring at the same time, and control the pH value to 11.0, and react for 13.5 hours to obtain the particle size (D 50 ) is 7.5 kg of co-precipitated precursor particles of copper nickel hydroxide of 11.4 μm.
[0027] The prepared precursor particles were mixed with 5.6kg lithium nitrate (LiNO 3 ) were fully mixed and baked at a temperature of 700° C. for 16 hours to obtain copper (II) nickel (II) acid lithium (Li 2 Cu 0.86 Ni 0.14 o 2 )solid. Break the solid into powder and put it into 10L of liquid bromine-acetonitrile solution with a concentration of 2.5% by volume, wash and dry it after oxidation for 5 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com