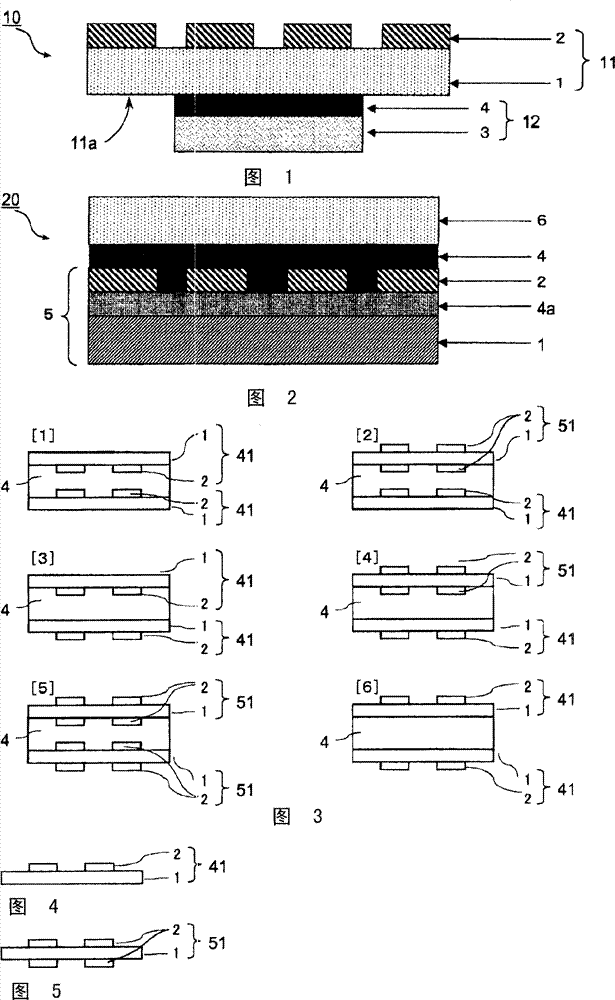
Bonding agent composition, bonding agent tablet using same and its uses
A technology of adhesives, compositions, applied in the direction of film/sheet adhesives, adhesives, adhesive types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0311] Next, the present invention will be described in more detail by showing examples, but the present invention is not limited thereto. In addition, in the examples, parts and % mean parts by weight and % by weight, respectively, Mn means number average molecular weight, and Mw means weight average molecular weight.
Synthetic example 1
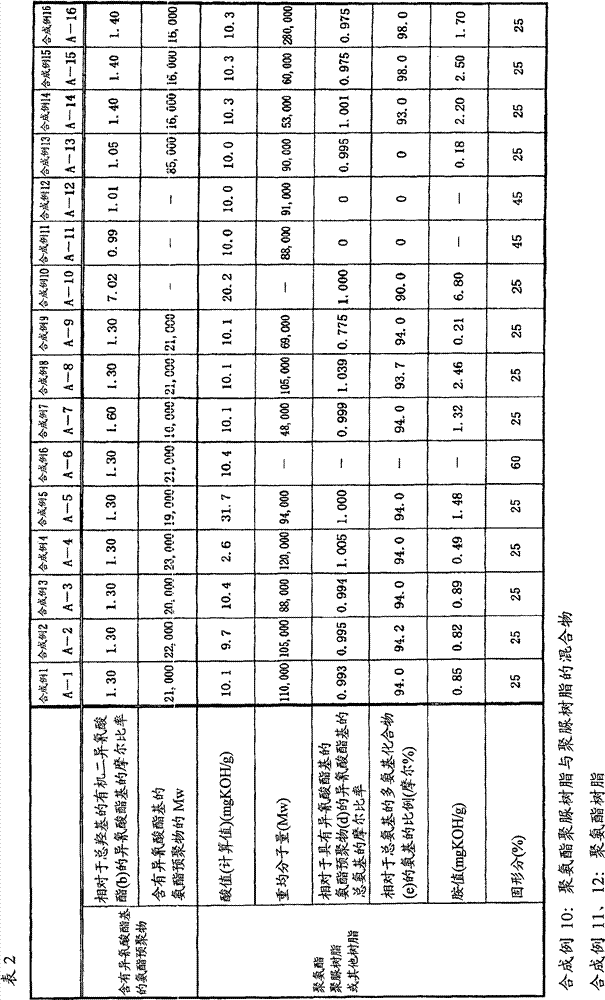
[0313] In a reaction vessel equipped with a stirrer, a thermometer, a reflux cooler, a dropping device, and a nitrogen gas introduction pipe, the polyester polyol obtained from terephthalic acid, adipic acid, and 3-methyl-1,5-pentanediol was charged. 195.2 parts of alcohol (Kuraray Polyol P-2011 manufactured by KURARAY Co., Ltd., Mn=2040), 6.67 parts of dimethylol butyric acid, 40.7 parts of isophorone diisocyanate, and 70.0 parts of toluene, at 90°C under a nitrogen atmosphere React for 4 hours, add 250 parts of toluene therein, and obtain the urethane prepolymer (Mw=21,000; The molar ratio of the isocyanate group of the diisocyanate (b) is 1.30) solution.
[0314]Then, in a mixture of 6.08 parts of isophorone diamine, 0.59 parts of di-n-butylamine, 112.5 parts of 2-propanol and 184.5 parts of toluene, 506.3 parts of the above-mentioned urethane prepolymer solution containing isocyanate groups were added. React at 85°C for 4 hours, dilute with 63.0 parts of toluene and 27.0 ...
Synthetic example 2~5、7~9、13 and 16
[0316] Except having used the raw material shown in Table 1, it reacted similarly to the synthesis example 1, and obtained the solutions A-2-A-5, A-7-A-9, A-13, and A-16 of polyurethane polyurea resin. The properties of the obtained polyurethane polyurea resin are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| amine value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com