Anthracene nucleus medicament nano-preparation, preparation method and application thereof
A nano-preparation and anthracycline technology, applied in the field of nano-preparation and its preparation, can solve the problems of high preparation conditions, expensive preparation equipment, QCQA is difficult to control, etc., to reduce costs, enhance anti-tumor activity, easy The effect obtained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
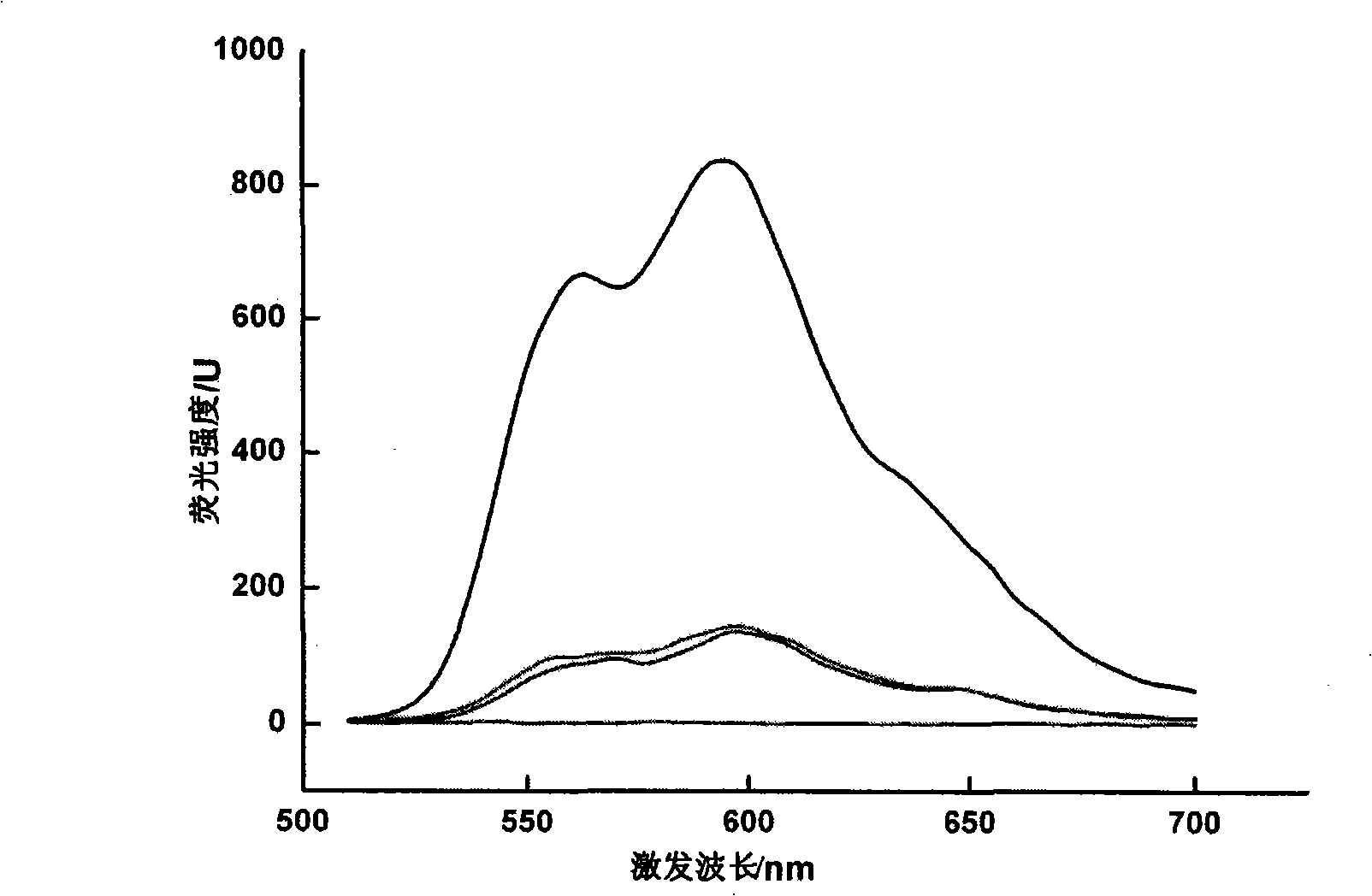
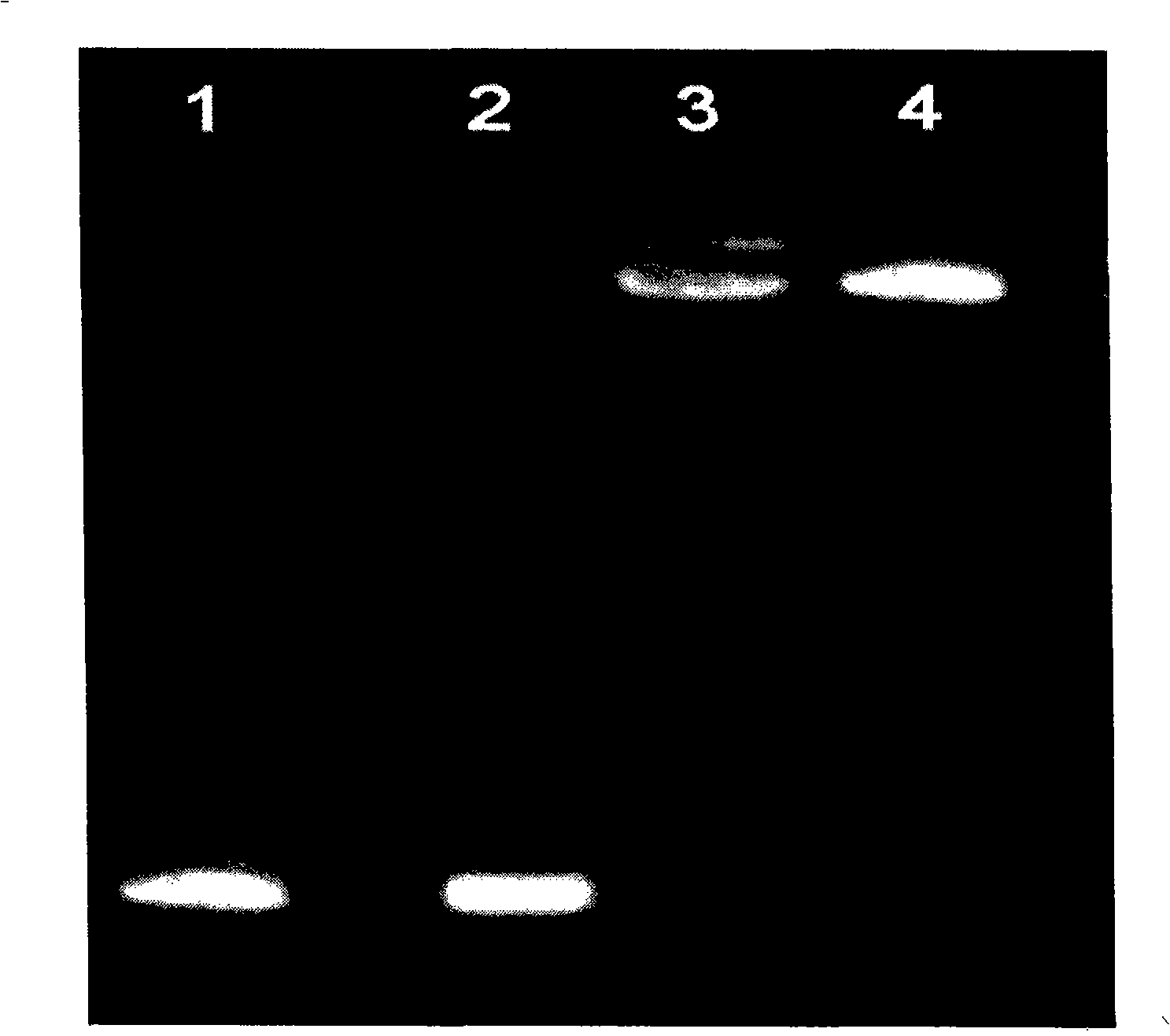
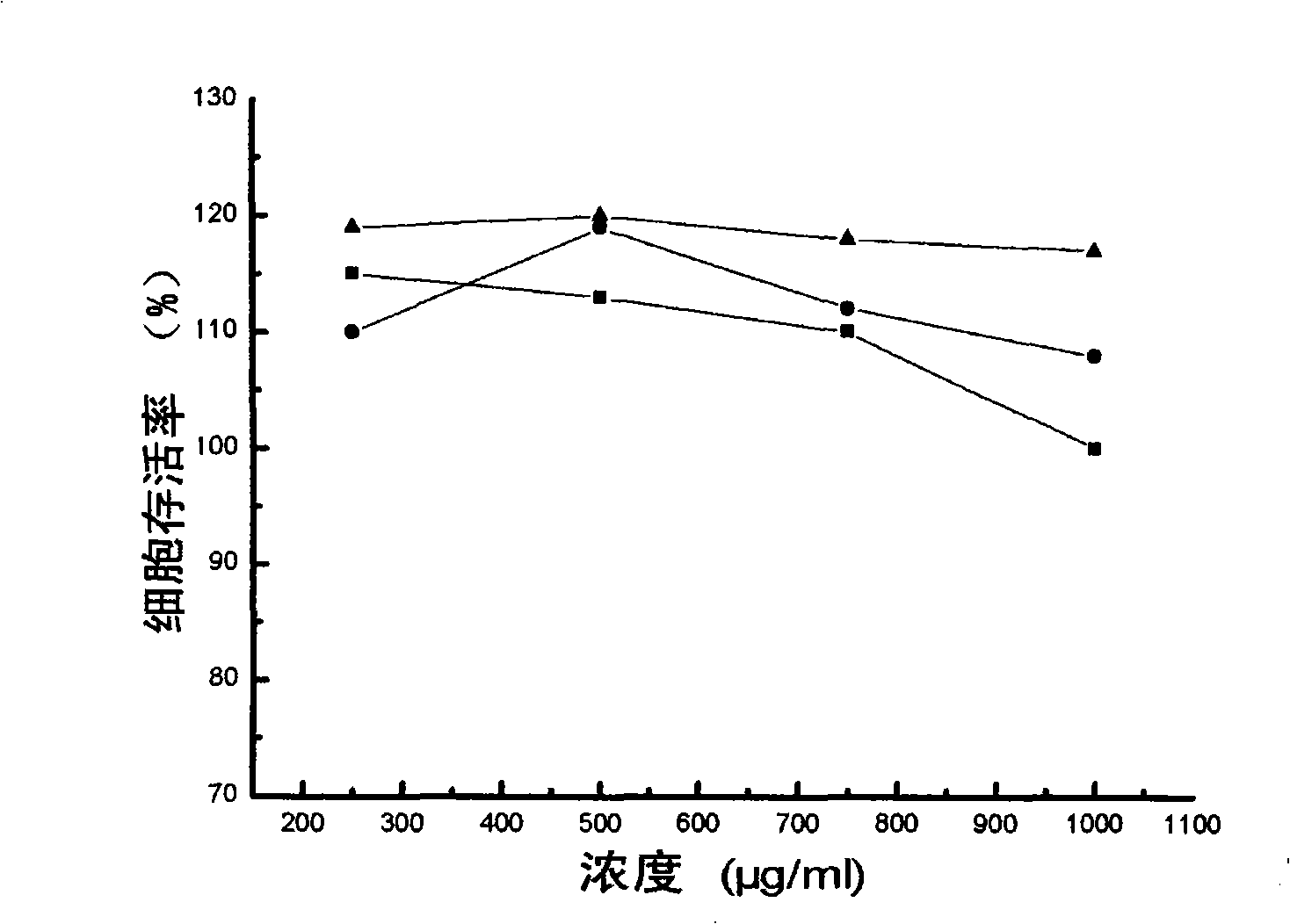
[0026] According to the present invention, it is related to the effective compounding of anthracyclines and nucleic acids and then compounding with cationic compounds. In the specific implementation process, the cationic compounds we mainly investigate mainly include chitosan, polyethyleneimine (polyethyleneimine, PEI), polylysine amino acid (poly-L-lysine PLL), polyhistidine, polyarginine, polydimethylaminoethylmethacrylate (poly(2-(dimethylamino)ethylmethacrylate), pDMAEMA), and cationic Gelatin; the tested nucleic acids include deoxyribonucleic acid (DNA) and ribonucleic acid (RNA); the tested anthracyclines mainly include daunorubicin (daunorubicin, daunorubicin, daunomycin, DNR), doxorubicin (doxorubicin) , DOX, doxorubicin, adriamycin, ADM), epirubicin (epirubicin, EPI) and idarubicin (idarubicin, demethoxydaunorubicin, demethoxydaunorubicin, IDA) and synthetic mitoxantrone (mitoxatrone). The specific combination is epirubicin-DNA-cationized gelatin, daunubicin-DNA-chit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com