Bis-quaternary ammonium salt cationic surfactant, preparation and use thereof
A technology of surfactant and double quaternary ammonium salt, which is applied in the application of oilfield fungicides, and the field of preparation of Gemini quaternary ammonium salt cationic surfactant, can solve the problems of drug resistance, high cost, and complicated preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
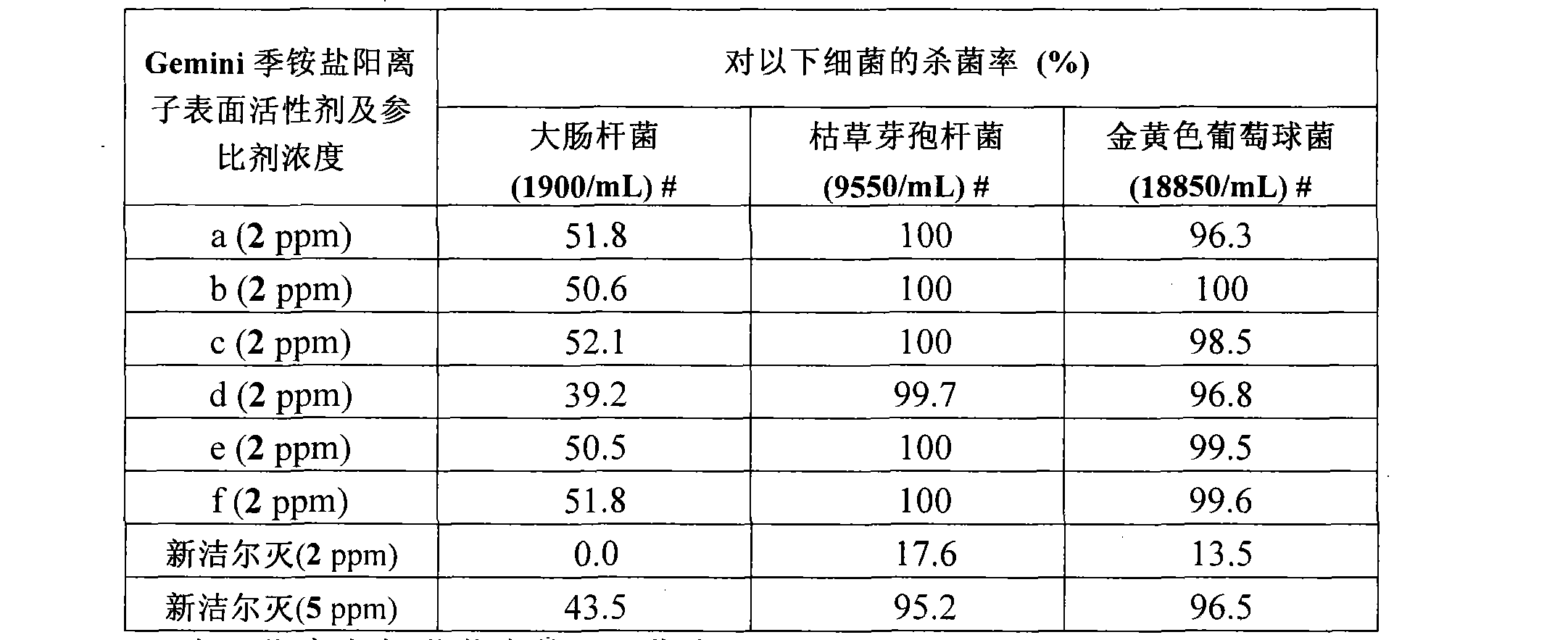
Embodiment 1
[0068] Example 1: N, N, N', N'-tetramethyl-N, N'-didodecyl-1,2-xylylene diammonium dibromide (1,2-BGBn) Synthesis:
[0069] Accurately measure 2.8mL (density=0.76g / mL, 0.01mol) of N,N-dimethyldodecylamine, weigh 1.06g (0.004mol) of o-dibromomethylbenzene, put the above reagent in 100mL In the round bottom flask, add 5mL ethanol in the reflux device, heat to reflux until a light yellow solid is produced, remove the ethanol by rotary evaporation, add 10mL CH 2 Cl 2 Solvent, to dissolve the solidified reactant as much as possible, filter out the insoluble matter, and place the filtrate to precipitate 1.46 g of transparent flaky crystals (52.8% yield), with a melting point of 54-56°C.
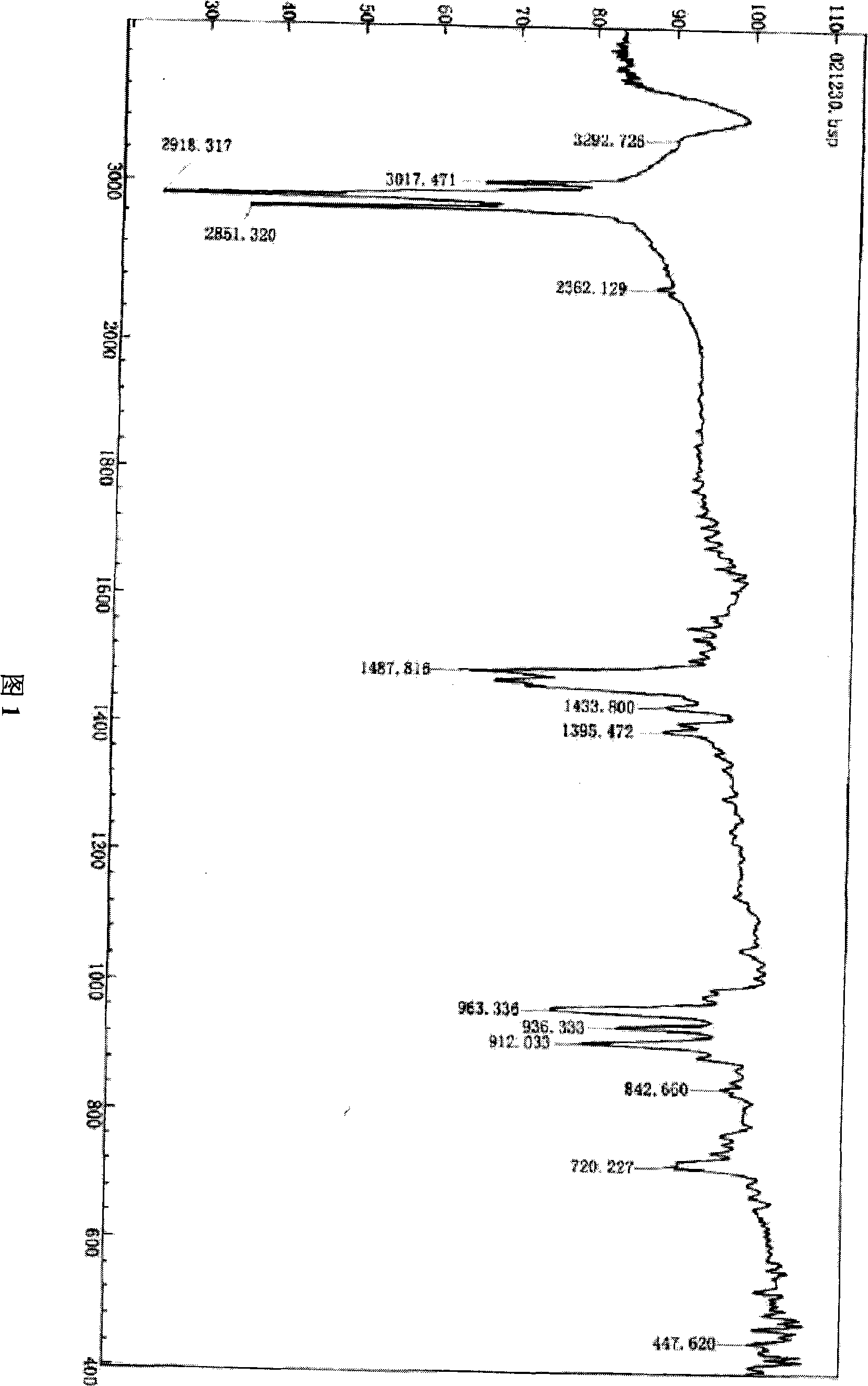
[0070] Infrared analysis results: the main infrared absorption peaks are:
[0071] IR (KBr, cm -1 ): 3016(m), 2917(v), 2852(m), 1486(m), 1450(m), 1433(w), 1395(w), 962(m), 935(m), 915(m ), 846(vs), 722(m), 448(w).
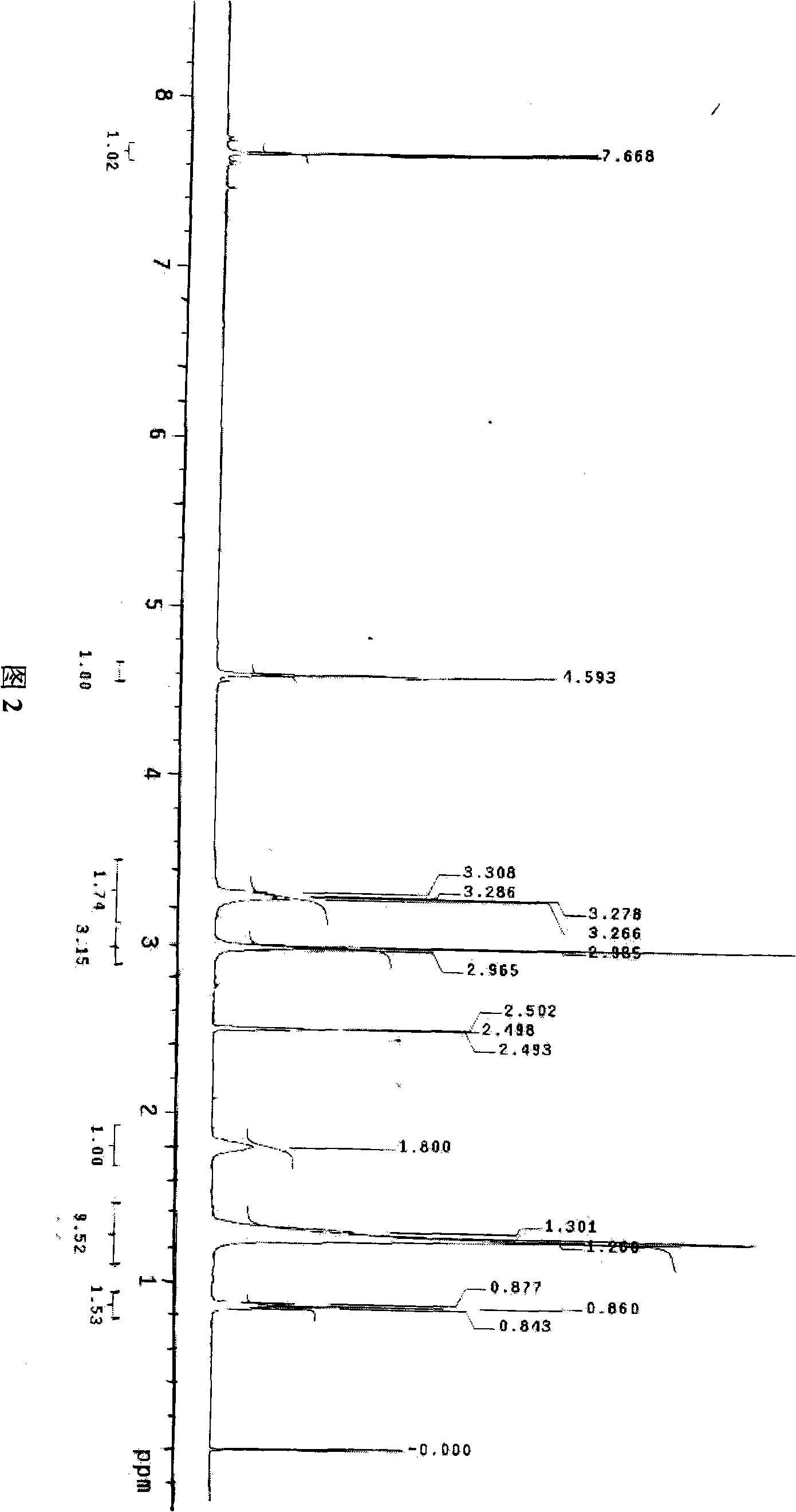
[0072] NMR:
[0073] 1 H NMR (CDCl 3, δ / ppm): 7.68 (s, 2H, Ph-H), 7.70 (s,...
Embodiment 2
[0075] Example 2: N, N, N', N'-tetramethyl-N, N'-dodedecyl-1,3-xylylene diammonium dibromide (1,3-BGBn) Synthesis:
[0076] Weigh 1.06g (0.004mol) m-dibromomethylbenzene, add it to a round-bottomed flask filled with 10mL of acetone, and under magnetic stirring, mix 2.8mL (density=0.76g / mL, 0.01mol) N, N - Add dimethyl dodecylamine to it, and stir at room temperature for about 15 minutes to form a white solid. After continuing to stir for half an hour, filter under reduced pressure and dry to obtain 1.83 g of solid (yield 66.3%). The melting point of the compound was 225-227°C as measured by a melting point apparatus.
[0077] Infrared analysis results: the main infrared absorption peaks are:
[0078] IR (KBr, cm -1 ): 3012(w), 2955(m), 2925(s), 2858(m), 1466(m), 1380(w), 1335(w), 1262(w), 927(w), 832(w ), 750(w), 723(m).
[0079] NMR: 1 H NMR (CDCl 3 , δ / ppm): 7.91 (s, 2H, Ph-H), 7.78-7.76 (d, 2H, J=7.6HzPh-H), 7.62 (s, 1H, Ph-H), 4.68 (s, 4H, Ph-CH 2 -N + ), 3.35(s,...
Embodiment 3
[0081] Example 3: N, N, N', N'-tetramethyl-N, N'-dodedecyl-1,4-xylylene diammonium dibromide (abbreviated as 1,4-BGBn )Synthesis:
[0082] Weigh 1.06g (0.004mol) m-dibromomethylbenzene, add it to a round-bottomed flask filled with 10mL of acetone, and under magnetic stirring, mix 2.8mL (density=0.76g / mL, 0.01mol) N, N -Dimethyldodecylamine was added thereto, a white precipitate was produced immediately, and stirring was continued for 30 minutes. It was filtered under reduced pressure and dried to obtain 2.03 g of solid (yield 73.6%). The melting point of the compound was 224-226°C as measured by a melting point apparatus.
[0083] Infrared analysis results: the main infrared absorption peaks are:
[0084] IR (KBr, cm -1 ): 3010(m), 2922(v), 2858(m), 1485(m), 1465(m), 1402(w), 1226(w), 1005(w), 880(w), 825(w ), 720(m), 415(w).
[0085] NMR: 1 H NMR (CDCl 3 , δ / ppm): 7.67 (s, 4H, Ph-H), 4.59 (s, 4H, Ph-CH 2 -N + ), 3.27(s, 4H, N + -CH 2 ), 2.98(s, 12H, N + -CH 3 ), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com