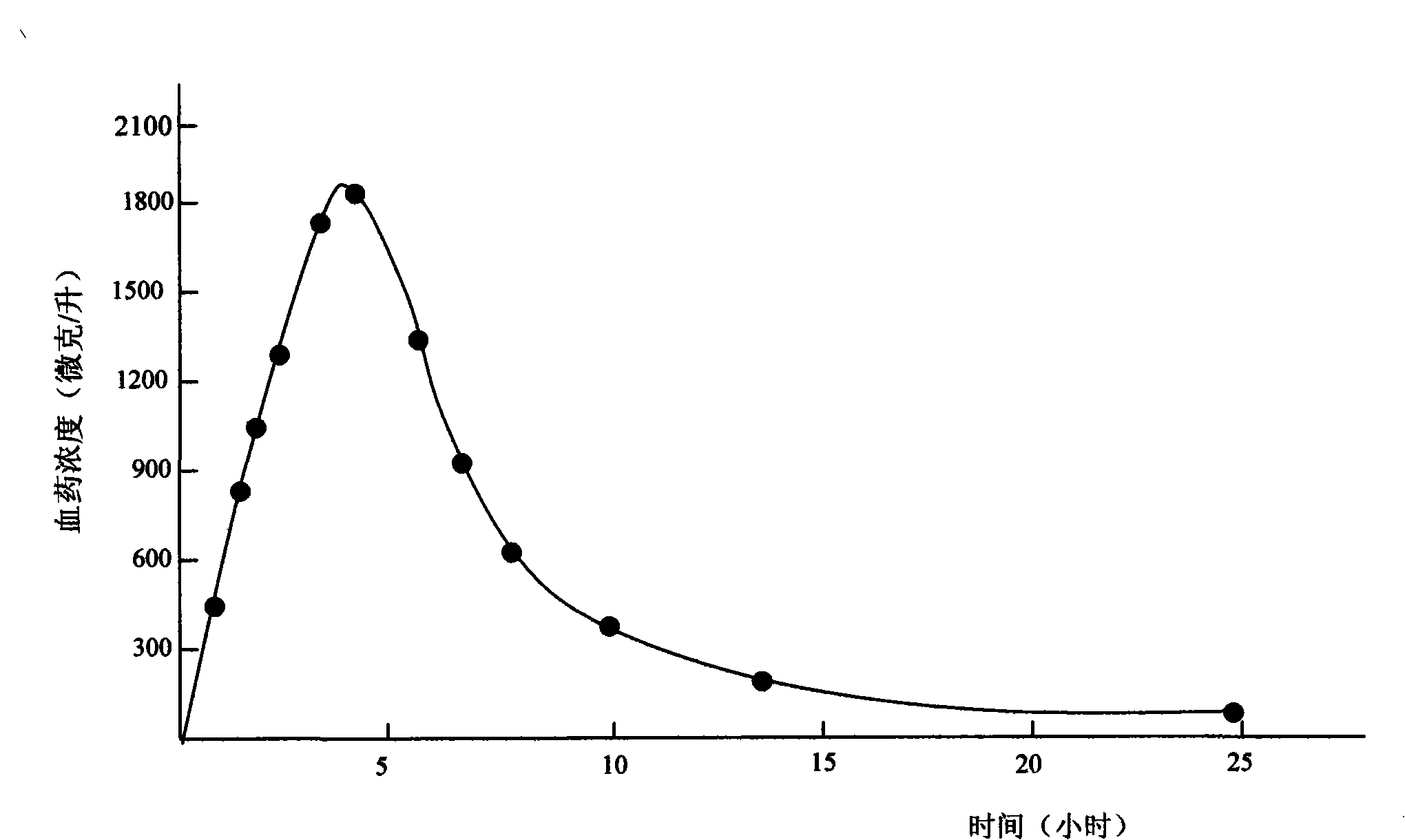
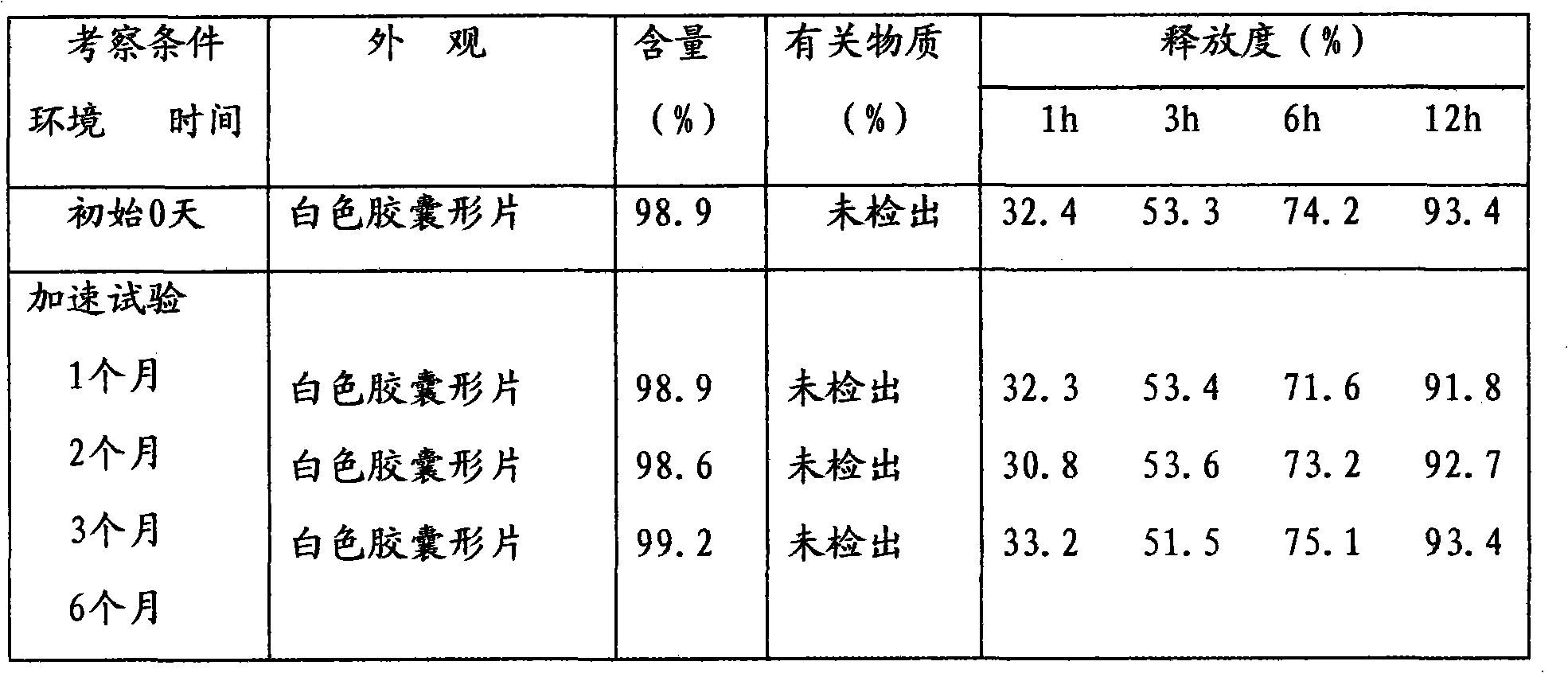
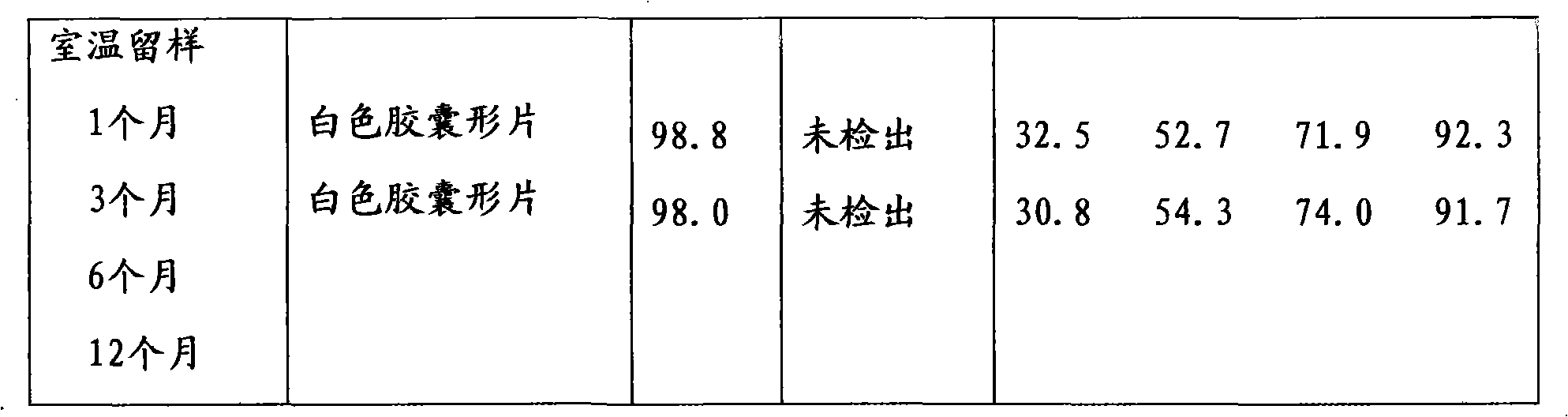
Use of the diabecron sustained-release tablet in treating the type 2 diabetes mellitus
A technology for metformin hydrochloride and type 2 diabetes, which is applied in the application field of metformin hydrochloride sustained-release tablets in the treatment of type 2 diabetes, and can solve the problems of high incidence of adverse reactions, large dosage, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The metformin hydrochloride sustained-release tablet, calculated on the basis of preparing 1000 tablets, contains 500 g of metformin hydrochloride, 420 g of hydroxypropyl methylcellulose, 30 g of sodium carboxymethyl cellulose, 20 g of microcrystalline cellulose, and 30 g of magnesium stearate.
Embodiment 2
[0066] The metformin hydrochloride sustained-release tablet, calculated on the basis of preparing 1000 tablets, contains 420 g of metformin hydrochloride, 490 g of hydroxypropyl methylcellulose, 50 g of sodium carboxymethyl cellulose, 20 g of microcrystalline cellulose, and 20 g of magnesium stearate.
Embodiment 3
[0068] The metformin hydrochloride sustained-release tablet, calculated on the basis of preparing 1000 tablets, contains 600 g of metformin hydrochloride, 340 g of hydroxypropyl methylcellulose, 20 g of sodium carboxymethyl cellulose, 20 g of microcrystalline cellulose, and 20 g of magnesium stearate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com