Preparation method of high intensity biodegradable supramolecule hydrogel
A supramolecular hydrogel and biodegradation technology, applied in the field of preparation of high-strength biodegradable supramolecular hydrogel, can solve the problems of low strength, inappropriateness, low strength, etc., and achieve a simple preparation method and fast application. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
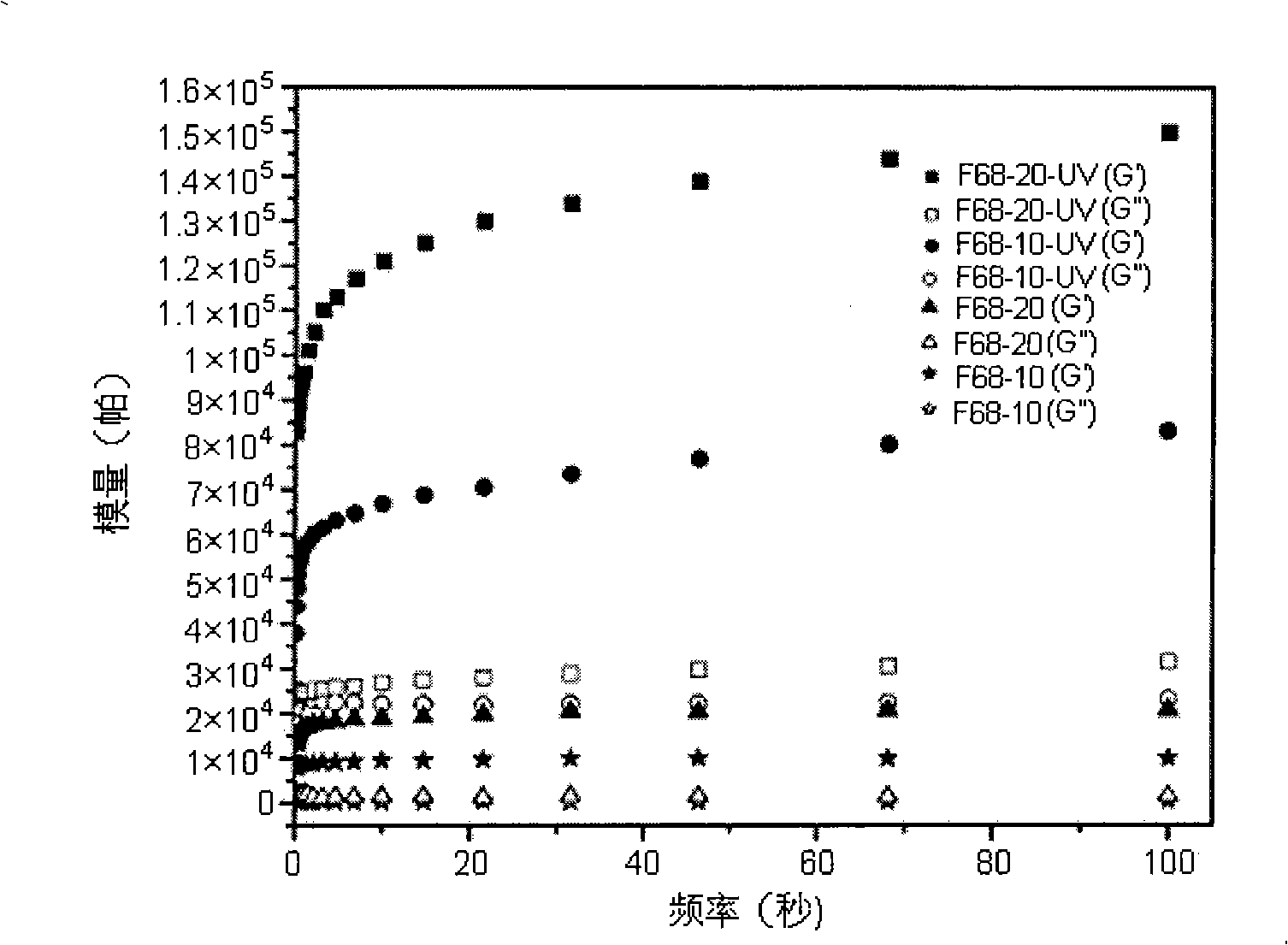
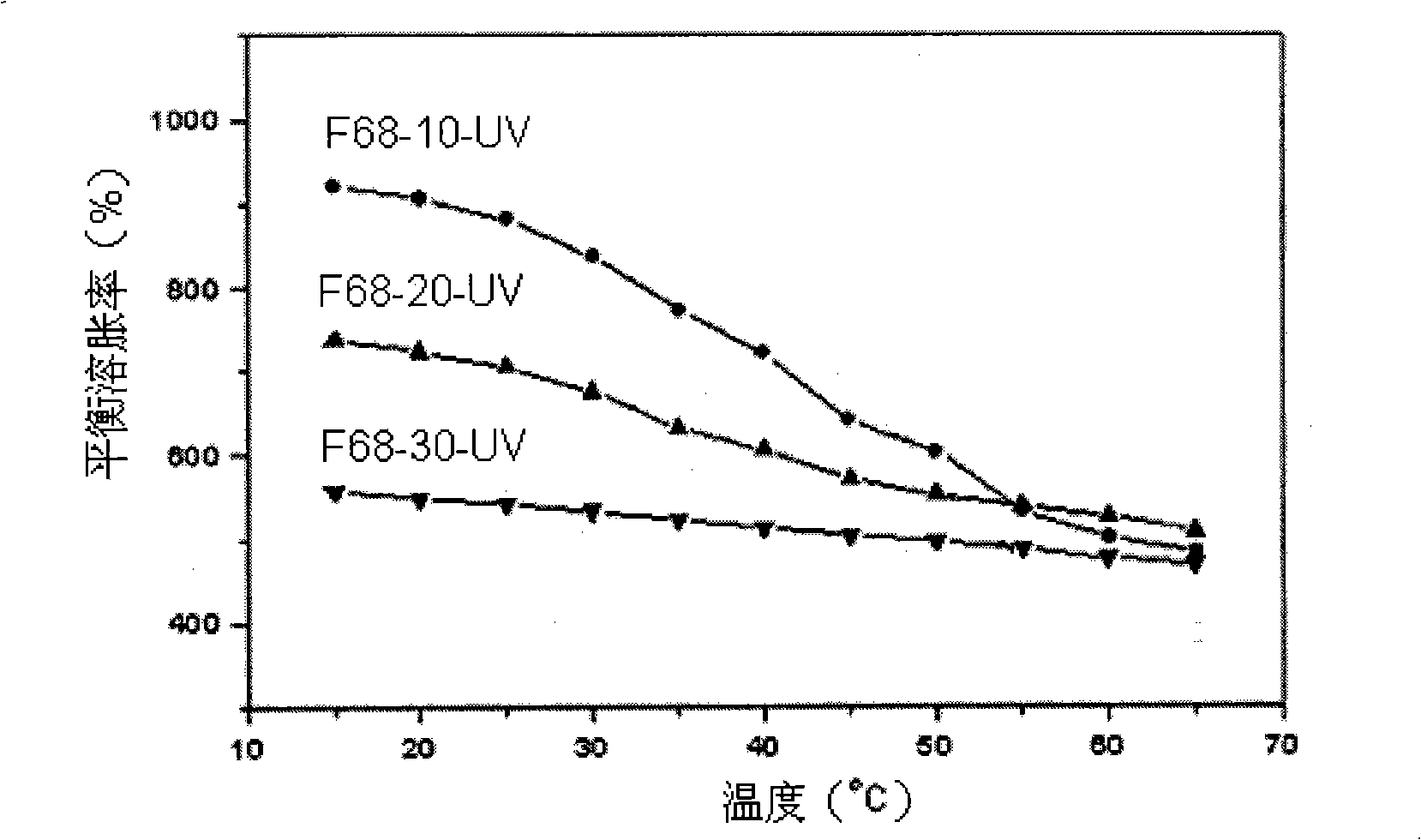
Image
Examples
Embodiment 1
[0019] Add 10g of dry F68 (a Pluronic block copolymer with an average molecular weight of 8400) to a 50ml clean single-necked round bottom flask, and add 1.09g of freshly distilled ε-caprolactone monomer and 3mg of For stannous octoate, after evacuating for 10 minutes, introduce argon gas, and repeat evacuation-ventilation for 3 times, and finally, under the protection of argon gas and magnetic stirring, the bulk polymerization reaction is carried out at 130°C for 12 hours, after cooling, it is dissolved in chloroform, and Precipitate with anhydrous ether, filter, and vacuum-dry the precipitate at 30°C to constant weight to obtain a water-soluble F68 / PCL block copolymer.
[0020]Add 5 g of the F68 / PCL block copolymer synthesized above to a 100 ml single-necked round-bottomed flask containing 60 ml of dry dichloromethane, place the single-necked round-bottomed flask in an ice bath and cool it to 0°C, and first add 0.5 ml of triethyl ether amine, then add 0.26ml of acryloyl chlo...
Embodiment 2
[0023] Add 60ml of toluene and 10g of F127 (a Pluronic block copolymer with an average molecular weight of 12600) to a 100ml clean single-necked round-bottomed flask, azeotropically distill 30ml of toluene under the protection of argon, and add 0.36g of toluene after cooling to room temperature Freshly distilled ε-caprolactone monomer and 10 mg stannous octoate were refluxed at 110°C for 18 hours under the protection of argon and magnetic stirring. Drying under vacuum to a constant weight yields a water-soluble F127 / PCL block copolymer.
[0024] Add 5 g of the F127 / PCL block copolymer synthesized above to a 100 ml single-necked round-bottomed flask containing 60 ml of dry dichloromethane, place the single-necked round-bottomed flask in an ice bath and cool it to 0°C, and first add 0.3 ml of triethyl ether amine, then add 0.22ml of methacryloyl chloride dropwise, react at 0°C for 12h, and then continue to react at 25°C for 12h, filter to remove by-products, and precipitate with...
Embodiment 3
[0027] Add 50ml of toluene and 10g of F68 (a Pluronic block copolymer with an average molecular weight of 8400) to a 100ml clean single-necked round-bottomed flask, azeotropically distill 25ml of toluene under the protection of argon, and cool to room temperature under argon Add 0.81g of freshly distilled ε-caprolactone monomer and 7mg of stannous octoate under protection, under the protection of argon and magnetic stirring, reflux reaction at 110°C for 24h, the reactant is cooled and precipitated with anhydrous ether, filtered , vacuum-dried to constant weight at 40°C to obtain a water-soluble F68 / PCL block copolymer.
[0028] Add 5 g of the F68 / PCL block copolymer synthesized above, 0.16 g of acrylic acid, 0.13 g of 4-methylaminopyridine and 0.34 g of N , N'-dicyclohexanecarbodiimide. The reaction system was reacted at 25° C. for 24 hours. After filtration, the filtrate was precipitated with petroleum ether, and the product was vacuum-dried at 40° C. to constant weight to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com