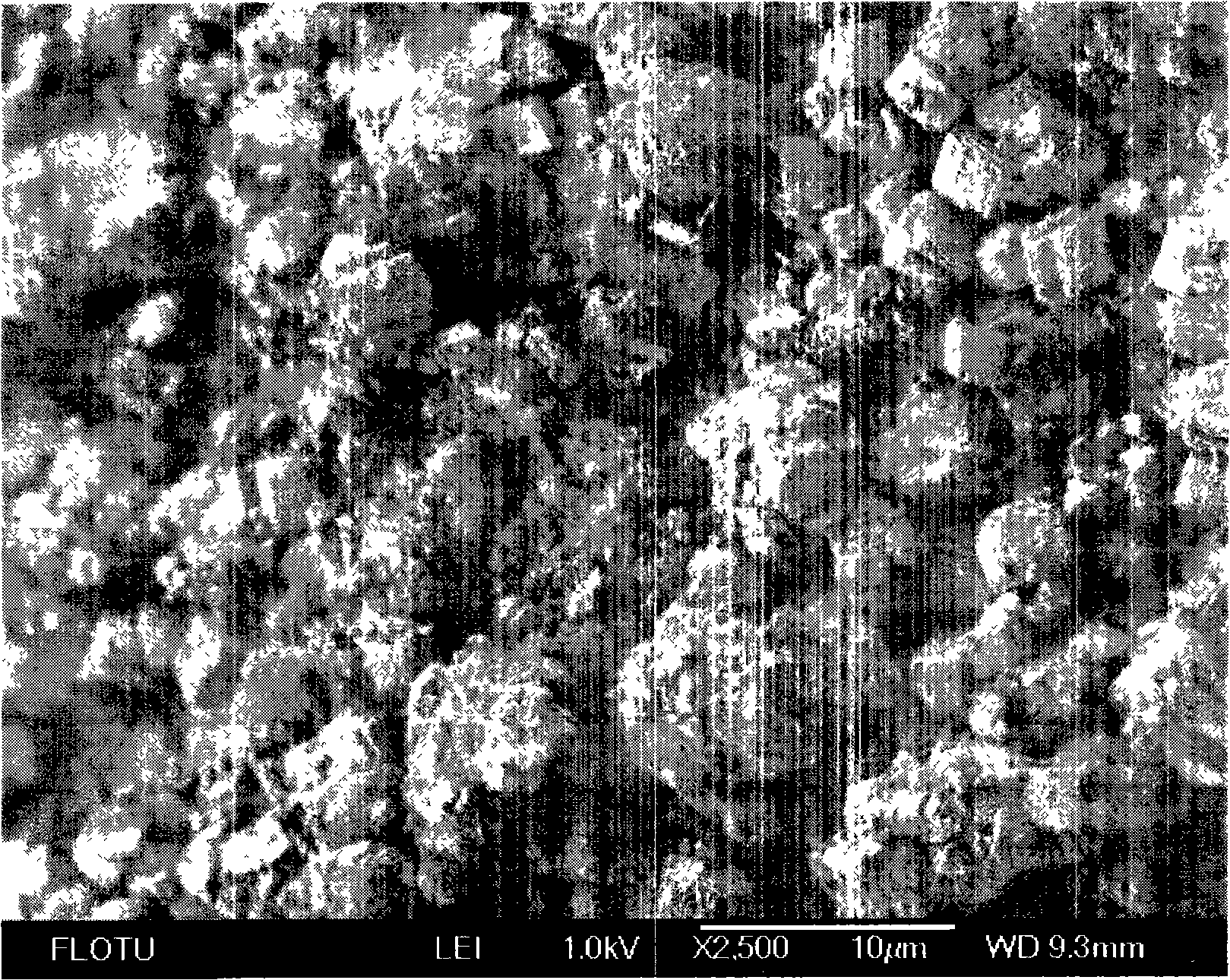
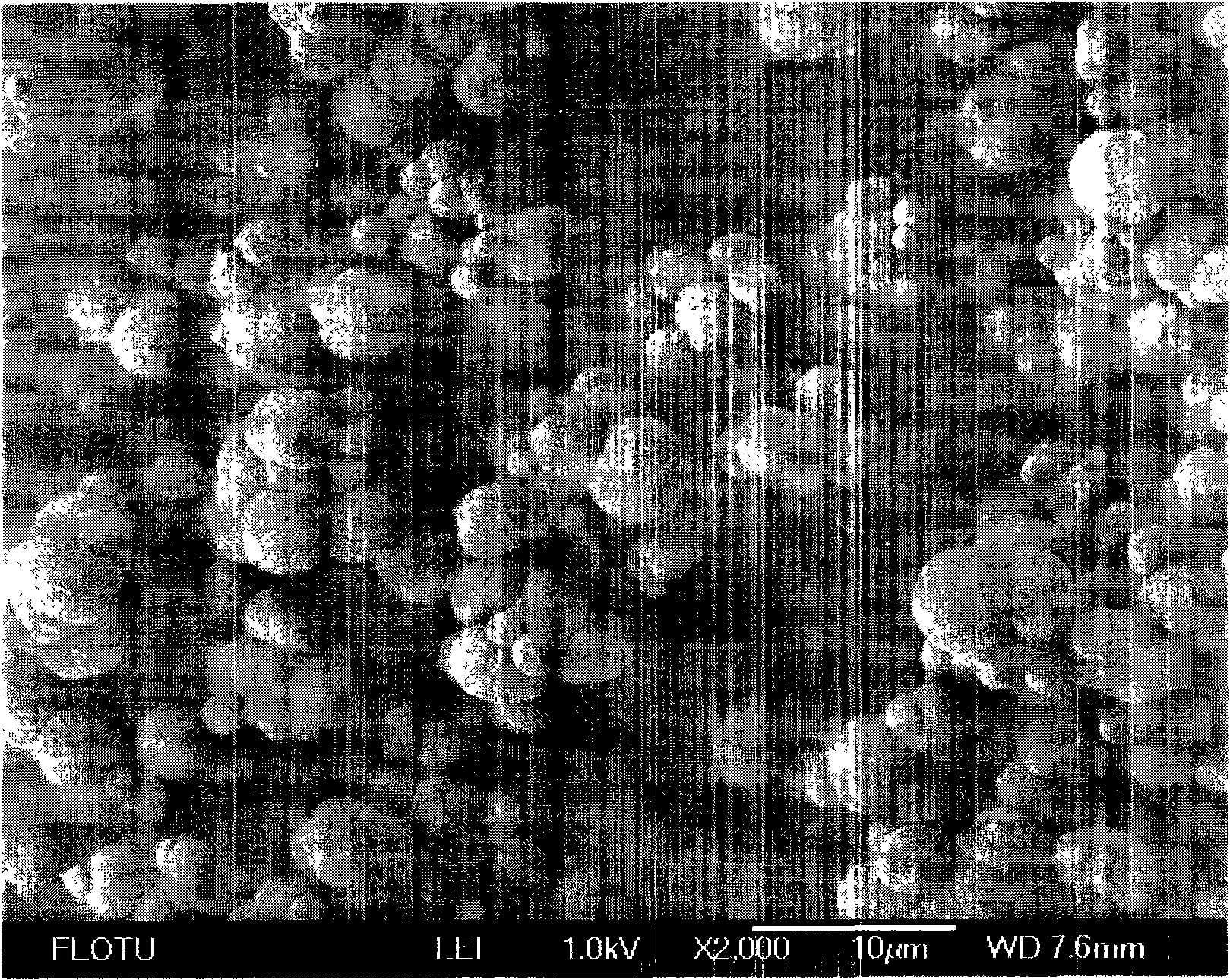
Novel technique for preparing fine calcium carbonate
A technology of fine calcium carbonate and amino acid calcium, applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problem of calcium carbonate quality, whiteness, poor crystal shape, decrease in porosity and specific surface area of carbide slag, and inability to remove impurities in carbide slag, etc. problems, to achieve the effect of low synthesis cost, utilization of three wastes, and high added value of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: The calcium glycinate extract generated by the reaction of glycine and carbide slag is carried out by intermittent bubbling carbonization to prepare fine calcium carbonate
[0039] 0.42L water is added in the reactor, then add 50g glycine, mix and stir, then add 32g calcium carbide slag (wherein calcium hydroxide content 77%), continue stirring at room temperature, finish after reaction 20min, filter and obtain 438g calcium glycinate liquid, Among them, the calcium ion concentration is 2.7%, and the calcium extraction rate is 93.5%. The extract is transferred to the intermittent bubbling reactor, and carbon dioxide is passed into the extract. When the pH=6.7, the reaction ends, and the filter drying obtains calcium carbonate 30.5g, and its aggregate diameter is distributed below 10 microns, and its purity is 96%. Whiteness 97%. The rest of the indicators are in line with the requirements of HG2226-91 indicators.
Embodiment 2
[0040] Embodiment 2: The calcium glycinate extract that generates by the reaction of glycine and carbide slag carries out kettle type carbonization method to prepare fine calcium carbonate
[0041] Add 0.5L of water into the reactor, add 150g of glycine, mix and stir, then add 80g of calcium carbide slag (calcium hydroxide content 77%) and continue to stir at room temperature, and finish the reaction after 20min to obtain 640g of calcium glycine extract, in which calcium The ion concentration is 4.76%, and the calcium extraction rate is 94%. The extract is transferred to a tank reactor, feeds carbon dioxide gas, and when the pH=7.6, the reaction ends, and the filtration and drying obtains calcium carbonate 50g, and its particle diameter is all below 10 microns, and its purity is 96%, and the whiteness is 96%. The rest of the indicators are in line with the requirements of HG2226-91 indicators.
Embodiment 3
[0042] Example 3: The calcium glycinate extract generated by the reaction of glycine and calcium carbide slag is passed through carbon dioxide in an inner circulation air-lift ultrasonic reactor to obtain fine calcium carbonate
[0043] Add 2.0L of water into the reaction kettle, then add 225g of glycine, heat to 40°C, and stir until dissolved. Then add 200g of calcium carbide slag (wherein calcium hydroxide content is about 77%) and continue to insulate and stir at 40°C, finish after reacting for 20min, and filter while hot to obtain 2240g of calcium glycinate extract, wherein the calcium ion concentration is 3.46%, and the calcium extraction rate is 94%. . The extract is transferred to the inner circulation air-lift ultrasonic reactor, and carbon dioxide is introduced. When the pH=7.2, the reaction ends, and the filtered and dried calcium carbonate 114g is obtained. The particle diameter is all below 10 microns, and most of them are distributed in the 1-3 microns, its purit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com