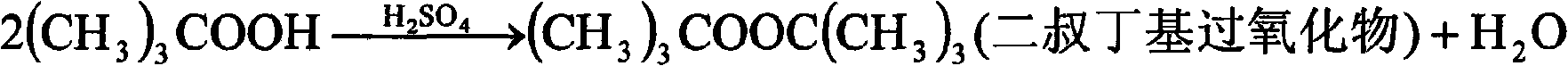Preparation of tert-butyl hydrogen peroxide and di-tert-butyl peroxide
A technology of di-tert-butyl peroxide and tert-butyl hydroperoxide, applied in the direction of organic chemistry, can solve the problems of high production cost, low yield, long process route, etc., and achieve environmental protection, high yield, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Mix 100g of 35% hydrogen peroxide, 30g of 98% sulfuric acid, 0.5g of phosphotungstic acid, 2g of ethylenediaminetetramethylenephosphonic acid, and 2g of hydroxyethylidenediphosphonic acid at 15°C to prepare a mixed solution.
[0040] Pour the above mixed solution into a three-neck flask, then add 75 g of tert-butanol while stirring, raise the temperature to 60° C., and keep the temperature for 30 minutes to obtain a crude product.
[0041] The crude product was separated with a separatory funnel to obtain 88.9 g of an oil phase. Through chromatographic analysis, it is detected that the content of tert-butyl hydroperoxide in the oil phase is 45.2%, the content of di-tert-butyl peroxide is 54.60%, and the others are 0.2%.
[0042] The above-mentioned oil phase is subjected to vacuum rectification at an absolute pressure of 2.5 to 10Kpa and a temperature of 20 to 50°C. The product at the top of the tower is condensed and weighs 40.2g. Chromatographic analysis shows that th...
Embodiment 2
[0044] Mix 100g of 35% hydrogen peroxide, 30g of 98% sulfuric acid, 0.5g of phosphotungstic acid, 2g of ethylenediaminetetramethylenephosphonic acid, and 2g of hydroxyethylidenediphosphonic acid at 15°C to prepare a mixed solution.
[0045] Pour 75 g of tert-butanol into a three-neck flask, then add the above mixed solution while stirring, raise the temperature to 60° C., and keep it warm for 30 minutes to obtain a crude product.
[0046]The crude product was separated to obtain 86 g of oil phase, and the oil phase was analyzed by chromatography, and it was detected that the content of tert-butyl hydroperoxide was 31.6%, the content of di-tert-butyl peroxide was 67.60%, and the others were 0.8%.
[0047] The above oil phase is subjected to vacuum rectification at an absolute pressure of 2.5 to 10Kpa and a temperature of 20 to 50°C. The product at the top of the tower is condensed to 57.9g. Chromatographic analysis shows that the content of di-tert-butyl peroxide is greater than...
Embodiment 3
[0049] Mix 100g of 35% hydrogen peroxide, 30g of 70% sulfuric acid, 2g of sodium citrate, 1g of phosphotungstic acid, and 2g of hydroxyethylidene diphosphonic acid at 15°C to prepare a mixed solution.
[0050] Add 74 g of tert-butanol into a three-necked flask, then add the above mixed solution while stirring, raise the temperature to 50° C., and keep it warm for 60 minutes to obtain a crude product.
[0051] The crude product was separated with a separatory funnel to obtain 88 g of the oil phase. The oil phase is analyzed by chromatography, and it is detected that the content of tert-butyl hydroperoxide is 55.19%, the content of di-tert-butyl peroxide is 44.70%, and the others are 0.19%.
[0052] The above oil phase was subjected to vacuum rectification at an absolute pressure of 2.5-10Kpa and a temperature of 20-50°C. The product at the top of the tower condensed and weighed 47.5g g. According to chromatographic analysis, the content of di-tert-butyl peroxide was greater tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com