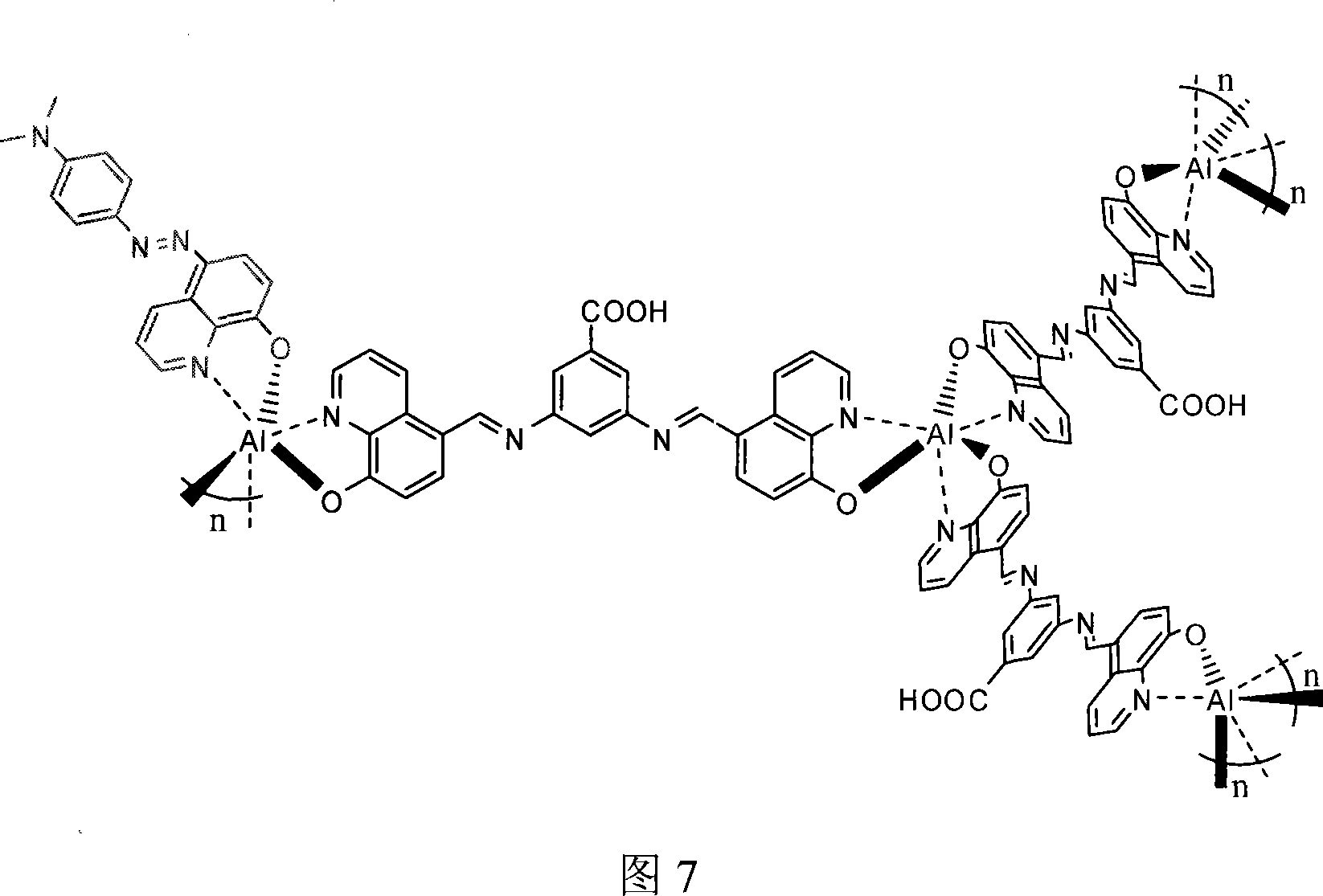
Preparation of doping optical non-linear chromophore coordination high polymer
An optical nonlinear and chromophore technology, which is applied in the field of preparation of doped optical nonlinear chromophore coordination polymers, can solve the problems of difficulty in single crystals and complicated preparation processes, and achieves low cost and simple doping method. , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 25 g of analytically pure 8-hydroxyquinoline in 100 mL of absolute ethanol, add NaOH solution (50 g of NaOH dissolved in 70 mL of deionized water), mix well and cool, then add dropwise chloroform (25 mL, drop over 1 hour). After the chloroform was added dropwise, the temperature was raised and stirred under reflux for 18 hours. Evaporate excess chloroform and ethanol on a rotary evaporator, dissolve the remaining residue in 600mL deionized water, adjust the pH value to 5-6 with dilute hydrochloric acid, a precipitate is formed, filter it with suction and wash it with deionized water, and remove the precipitate The material was dried in an oven at 80°C. Grind the dried solid into powder, extract with petroleum ether with a boiling range of 90-120°C for 48 hours in a fat extractor, cool the extract, collect the precipitate by filtration, and recrystallize with absolute ethanol. 2.1 g of 5-formyl-8-hydroxyquinoline are obtained. For mass spectrometric characteri...
Embodiment 2
[0035] 0.115g 3,5-diamino (8-hydroxyquinoline)-benzoic acid, 0.0182g5-(4-N, N dimethylaminobenzene) azo-8-hydroxyquinoline prepared by Example 1 and Add 0.06g of anhydrous ferric trichloride to 20mL of methanol, stir vigorously until the solution is clear.
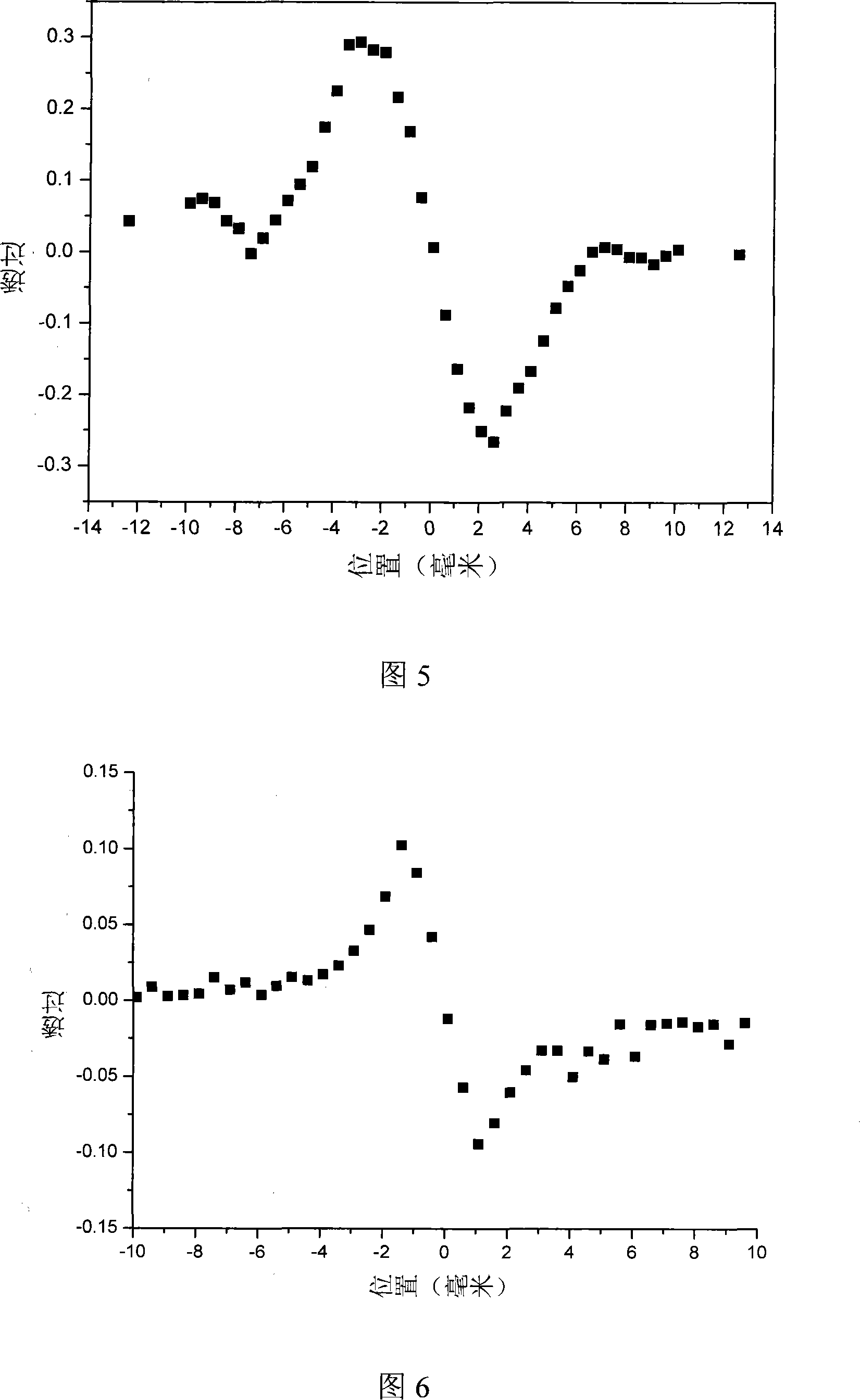
[0036] Films were prepared by spin coating method. Using Z-scan technology to obtain the nonlinear refractive index n of the material at a wavelength of 1064nm 2 =-1.84×10 -8 esu. See Figure 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com