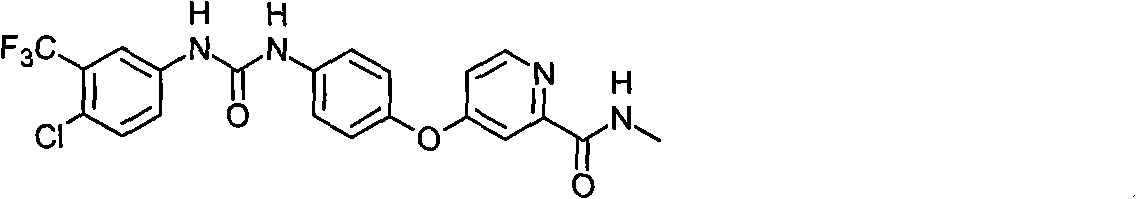
Entironment-friendly preparation of sorafenib intermediate
An environmentally friendly and intermediate technology, applied in the field of preparation of sorafenib intermediates, can solve environmental pollution and other problems, and achieve the effect of no waste gas emission and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
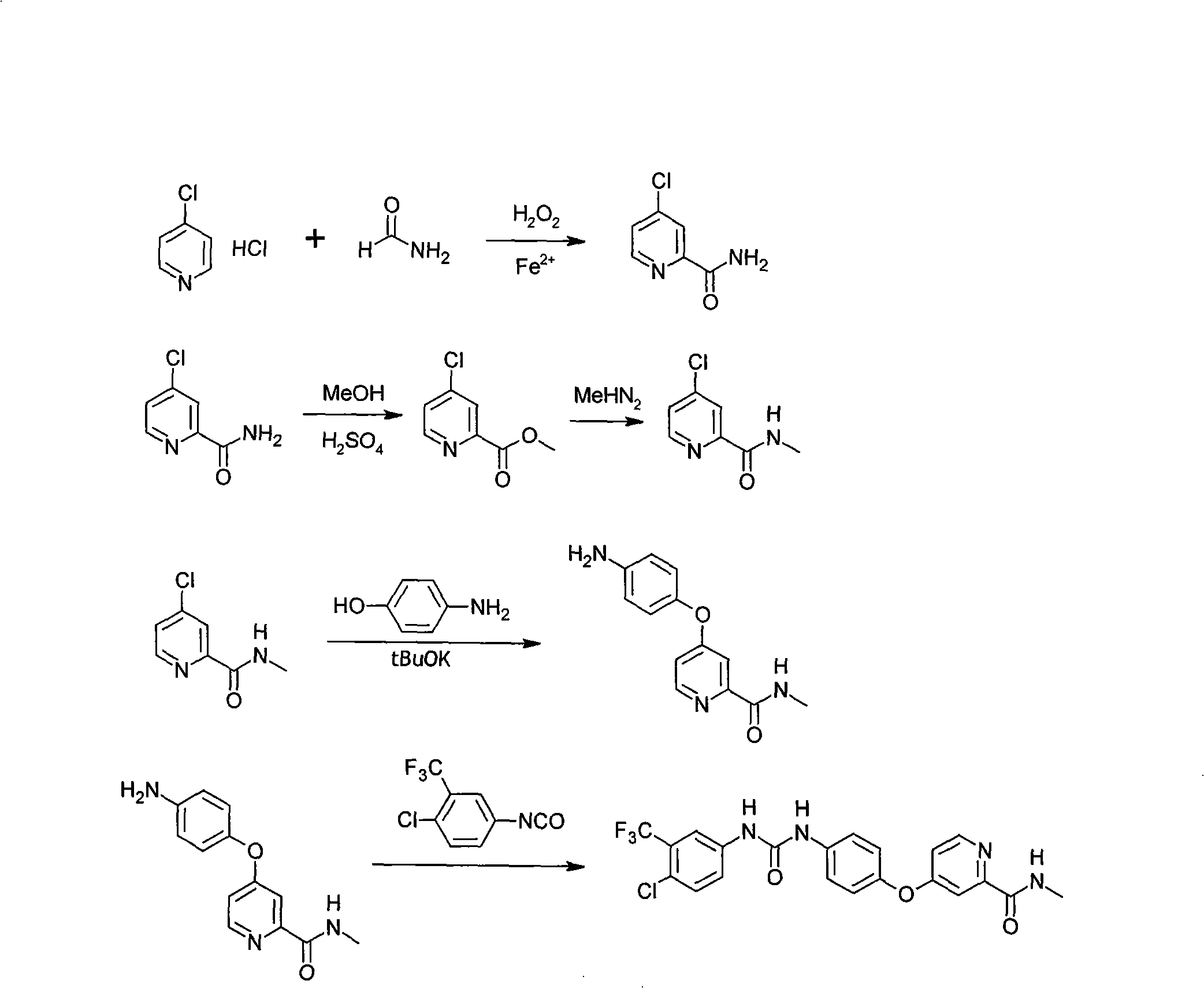
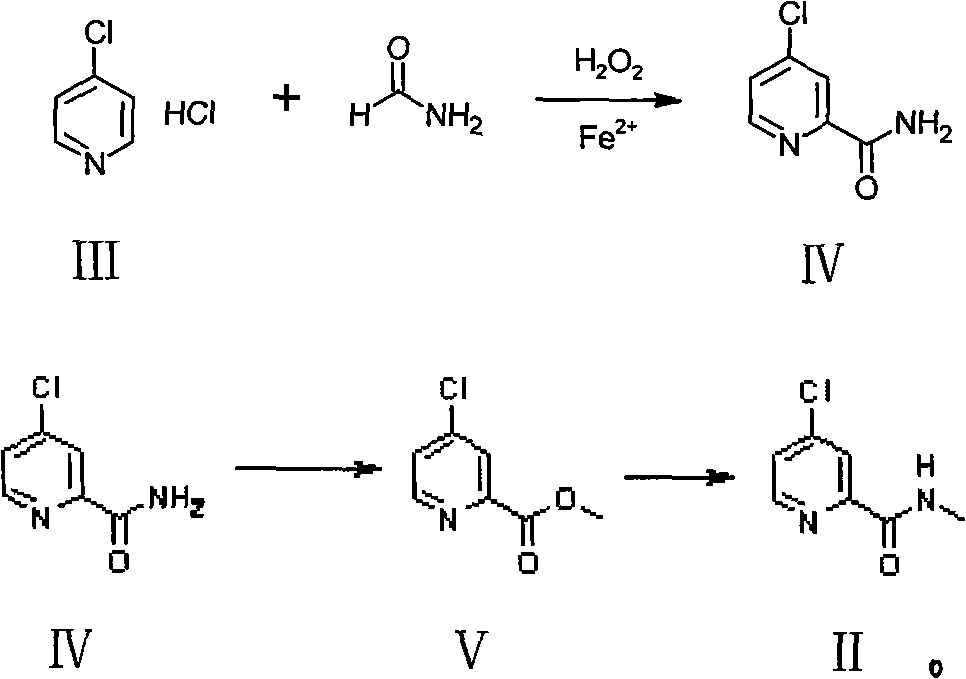
[0028] The preparation of embodiment N-methyl-(4-chloro-2-pyridyl) formamide
[0029] 1. Dissolve 4-chloropyridine hydrochloride in an appropriate amount of formamide, and slowly add 1.5 equivalents of hydrogen peroxide and 1.5 equivalents of ferrous sulfate heptahydrate at 10-15°C [temperature range 0°C-100°C] (both added at the same time , independently). After the reaction, excess formamide was distilled off, the residue was extracted with chloroform, washed with water and concentrated to dryness to obtain 4-chloropyridine-2-carboxamide.
[0030] 2. Dissolve 1.0 mol of 4-chloropyridine-2-carboxamide in an appropriate amount of anhydrous methanol, slowly add 1.02 mol of concentrated sulfuric acid dropwise, heat up and reflux until all raw materials disappear [reaction temperature 20-80°C]. Part of the methanol was recovered, and the reaction liquid was slowly added dropwise to the aqueous sodium bicarbonate / ethyl acetate two-phase system (sodium bicarbonate 1.2 mol) under s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com