Use of cyaniding 3-0 glucoside in preparing medicament or food for preventing and curing atrophic arthritis
A glucoside, rheumatoid technology is applied in the application field of cyanidin 3-O-glucoside in the preparation of rheumatoid arthritis drugs or food, and can solve the problem of large side effects and high incidence of drug-induced diseases problem, to achieve a good effect, good anti-inflammatory and analgesic, and inhibit the effect of adjuvant arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Maximum tolerated dose test and anti-inflammatory activity test of cyanidin 3-O-glucoside
[0020] 1. Test materials: Animals: ICR mice, 18-22g, both male and female; SD rats, weighing 180-200g, male, provided by Beijing Experimental Animal Research Center.
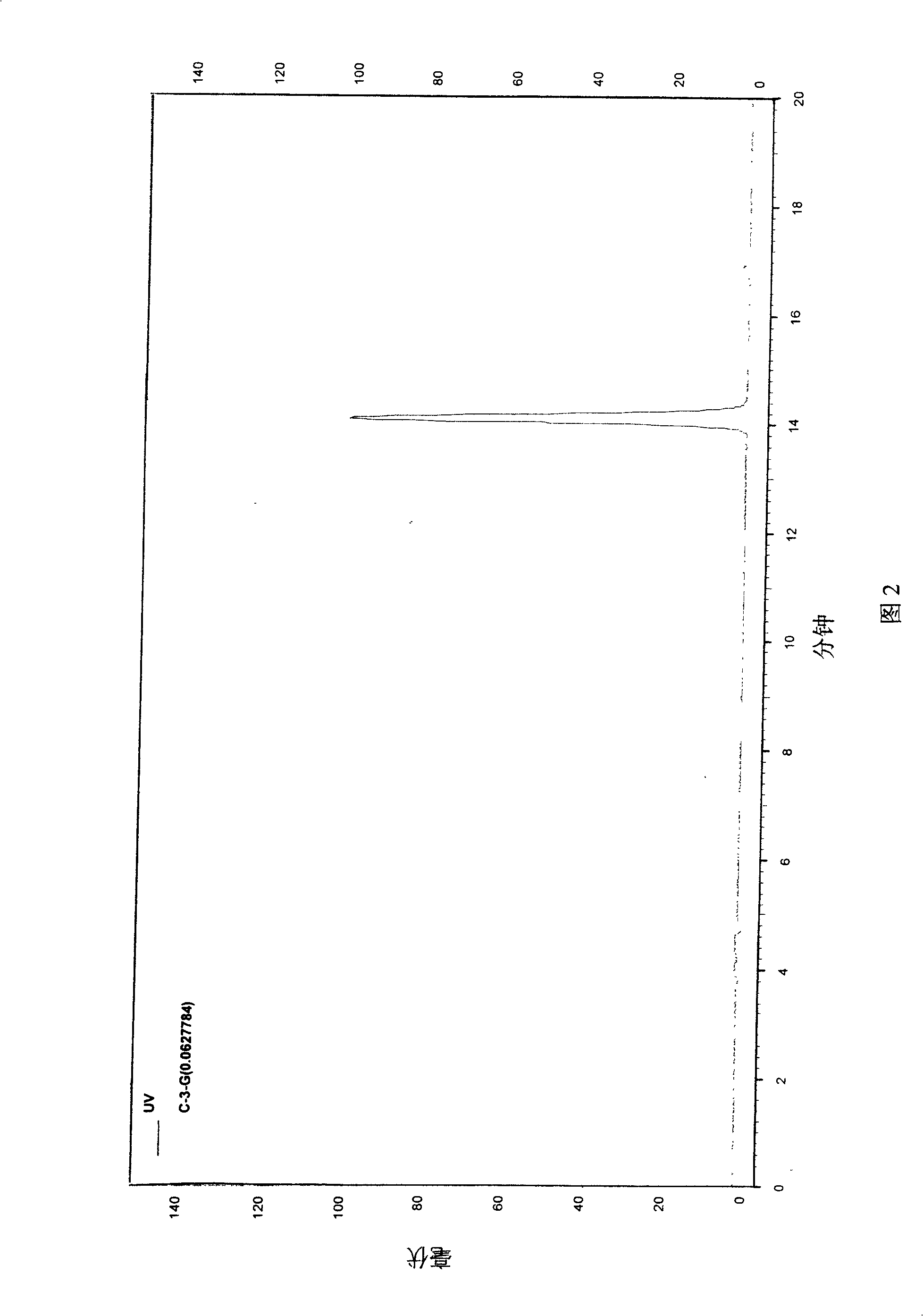
[0021] 2. Reagent: cyanidin-3-O-glucoside (hereinafter referred to as C3G, with a purity of 95%); aspirin was purchased from Hunan Yongke Light Industrial Products Import and Export Co., Ltd.; carrageenan was purchased from China Lianxing Industrial Co., Ltd. Company; electronic analytical balance, vortex mixer.
[0022] 3. Test method: (1) Maximum tolerance test: 40 ICR mice were divided into two groups, group 1, normal water control group; group 2, C3G group. There were 20 mice in each group, half male and half male. After fasting for 12 hours, the mice were intragastrically administered with a C3G solution with a maximum concentration of 0.4 g / mL within 1 day, once every 4 hours. A total of 3 times a day, 0.30...
Embodiment 2
[0032] Analgesic activity test of cyanidin 3-O-glucoside
[0033] 1. Test materials: Animals: ICR mice, 18-22g, both male and female; provided by Beijing Experimental Animal Research Center.
[0034] 2. Reagent: cyanidin 3-O-glucoside (C3G) (cyanidin 3-O-glucoside content 95%); aspirin was purchased from Hunan Yongke Light Industrial Products Import and Export Co., Ltd.; acetic acid was of analytical grade , electronic analytical balance, vortex mixer.
[0035] 3. Test method: (1) Acetic acid writhing test: 50 mice, half male and half male, were randomly divided into 5 groups, respectively administered with drugs or the same amount of water, and 1 hour after the drug, each mouse was intraperitoneally injected with 0.7% HAC physiological salt 0.2ml aqueous solution, observe the number of writhing reactions in mice within 20 minutes after injection of HAC, and calculate the analgesic inhibition percentage.
[0036]
[0037] (2) Mouse hot plate test: test according to Zhao Y...
Embodiment 3
[0046] Anti-adjuvant rheumatoid arthritis effect of cyanidin-3-O-glucoside
[0047] 1. Test materials: Animals: ICR mice, 18-22g, both male and female; SD rats, weighing 180-200g, male, provided by Beijing Experimental Animal Research Center.
[0048]2. Reagents: cyanidin-3-O-glucoside (purity: 95%); complete Freund's adjuvant, product of Sigma Company; dexamethasone injection, electronic analytical balance, vortex mixer.
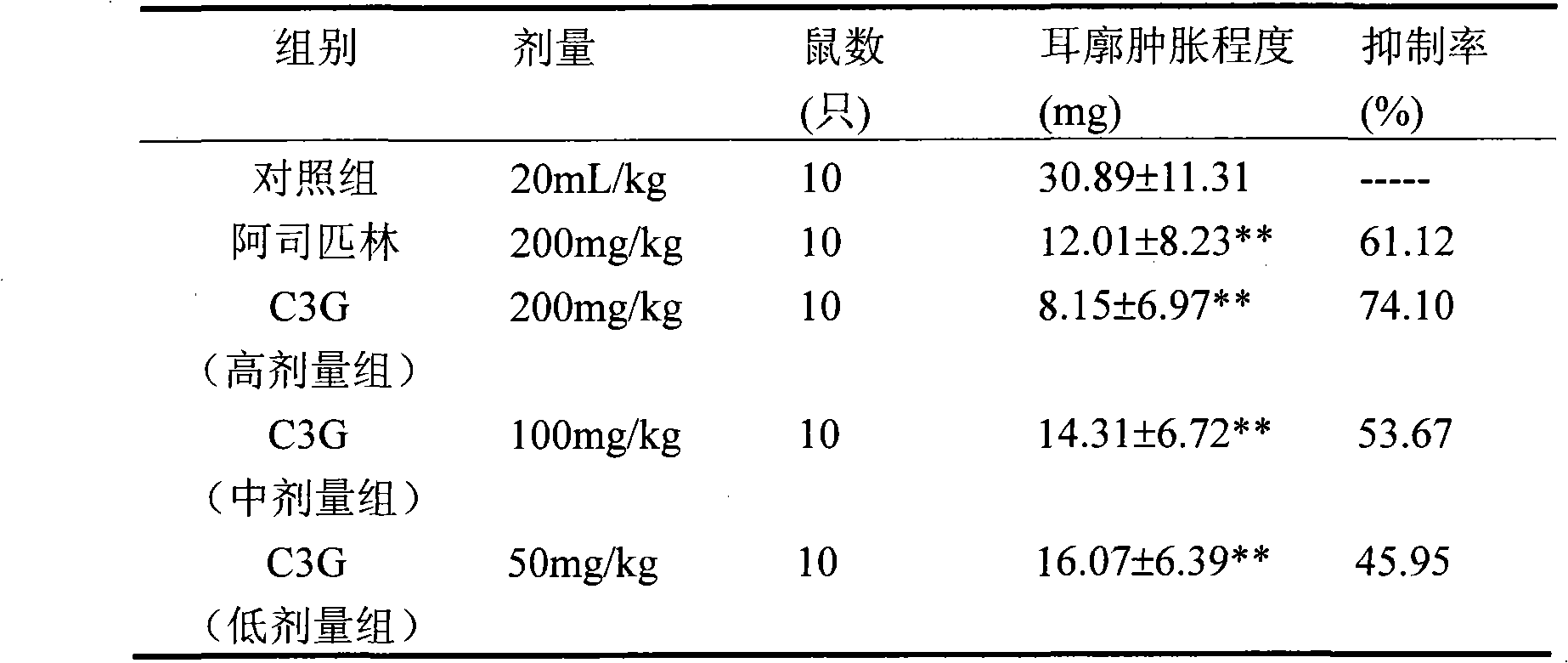
[0049] 3. Test method: get 48 male rats, body weight 160~180g, divide into 6 groups randomly according to body weight, be respectively control group (NS 10mI / kg); Model group (NS 10mI / kg); Dexamethasone group ( 0.85mg / kg); C3G medium and high dose (25, 50, 100mg / kg) groups. Each group was administered ig once a day for 24 consecutive days. After the start of the experiment, the volume of the left and right hind paws was measured by the volumetric method, and then 1 hour after administration on the first day, except for the control group, 0.1 mL of SC Freu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com