Method for preparing L-ascorbate-2-phosplate magnesium
A technology of ascorbic acid and monophosphate, applied in the field of preparation of L-ascorbic acid-2-monophosphate magnesium, can solve the problem of high purity of L-ascorbic acid-2-monophosphate magnesium, and achieve simple post-processing and environmental safety. Effect
Active Publication Date: 2008-11-19
WUXI KUAKE MICRONUTRIENT
View PDF2 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In view of the above problems, the present invention provides a preparation method of L-ascorbic acid-2-magnesium monophosphate, which does not need to use organic solvents in the preparation process, the post-treatment is simple, and will not cause environmental pollution problems, and the prepared Magnesium L-Ascorbyl-2-monophosphate is of high purity
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
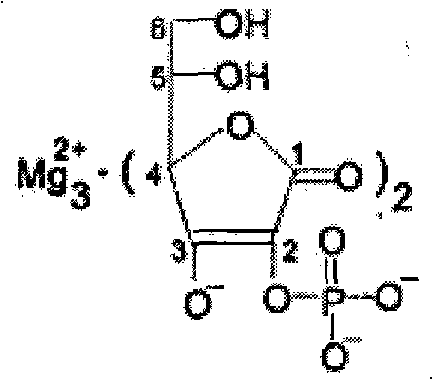
The invention relates to a preparation method for L-ascorbic acid-2-monophosphate ester magnesium. The method which adopts no organic solvent during the preparation process does not cause environmental pollution with simple after treatment and high purity of the prepared L-ascorbic acid-2-monophosphate ester magnesium. The method is characterized in that: water, L-ascorbic acid and calcium chloride are added into a reaction kettle with a temperature between -5 DEG C and 10 DEG C for agitation, then 20-60 percent of sodium hydroxide or calcium hydroxide aqueous solution is added slowly into the reaction kettle, a PH value is regulated to 6-9.5, and sodium trimetaphosphate with a dosage of 0.8-1.5 folds of vc is added into the reaction kettle with the temperature being kept between 15 DEG C and 35 DEG C; the reaction products are washed by water, the PH value is regulated to 0.5-3.0 through hydrochloric acid, sulphuric acid or oxalic acid, then filtration is carried out, and filtrate liquor passes through weak anion resin and is eluted in turn by 0.02m hydrochloric acid and 0.2-0.8m hydrochloric acid; magnesia is used to regulate the PH value of an eluted part which is filtrated to remove deposit, filtrate liquor is decompressed and condensed to 1 / 5 of the original volume at a temperature of 40 DEG C, ethanol with a volume being 2-4 folds of that of the condensed filtrate liquor and a content of 95 percent is added, and then crushing and drying is carried out after crystallization, centrifugal collection and washing.
Description
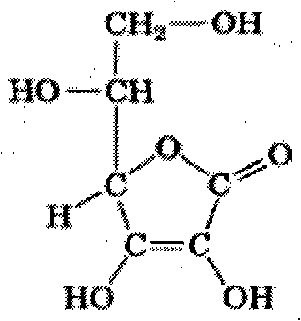
(1) Technical field The invention relates to a preparation method of L-ascorbic acid derivatives, in particular to a preparation method of L-ascorbic acid-2-monophosphate magnesium. (2) Background technology L-ascorbic acid (vitamin C, hereinafter referred to as VC) has a chemical structural formula as an acidic enol-structured lactone, which exists in fresh vegetables and fruits in nature, and is a kind necessary for the human body. vitamins. VC has been widely used in medicine, food, cosmetics and animal breeding and other fields. But VC its C 2 、C 3 The hydrogen of the enolate hydroxyl group is easy to dissociate, and it is easily oxidized into dehydrovitamin C by air and other oxidants, and will soon undergo an irreversible decomposition reaction and lose the VC reducing ability. It is found that the activity of VC is mainly derived from the two hydroxyl groups on its enol structure. If any of the hydroxyl groups is esterified with inorganic acids, the stability to h...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07F9/655
Inventor 汪中一冷胜利郁建兴
Owner WUXI KUAKE MICRONUTRIENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com