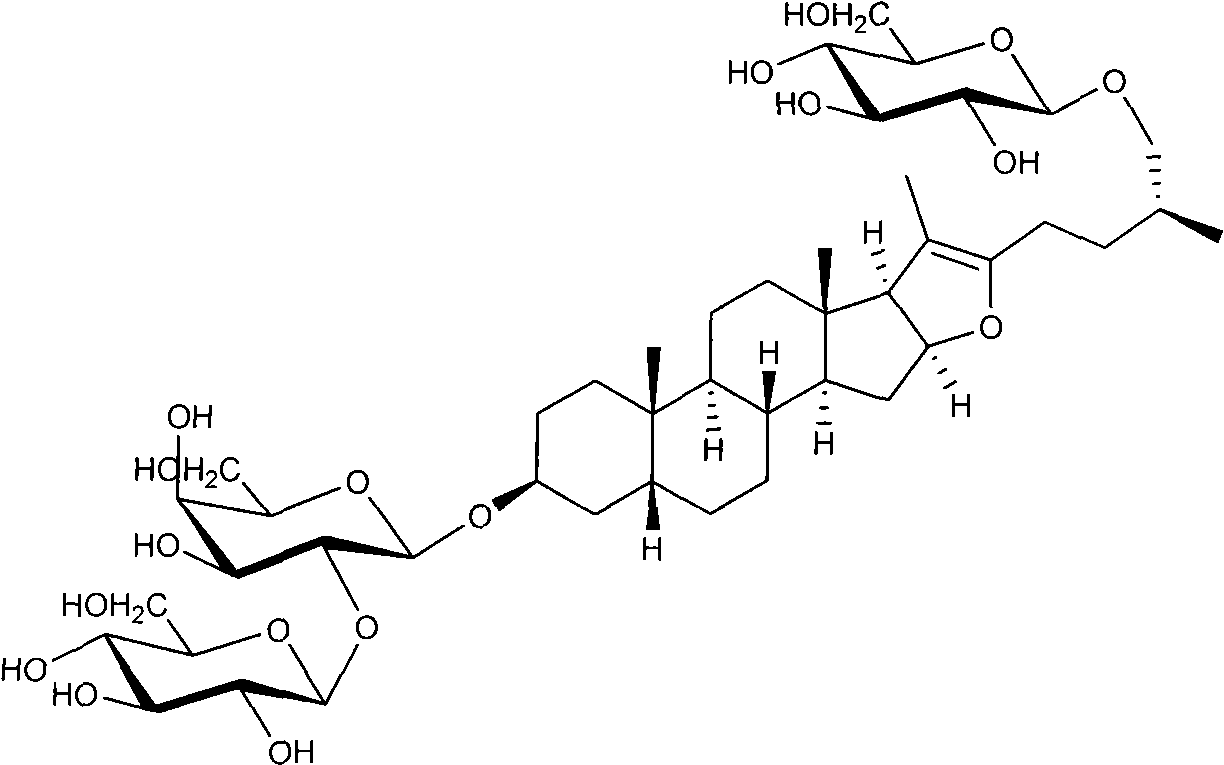
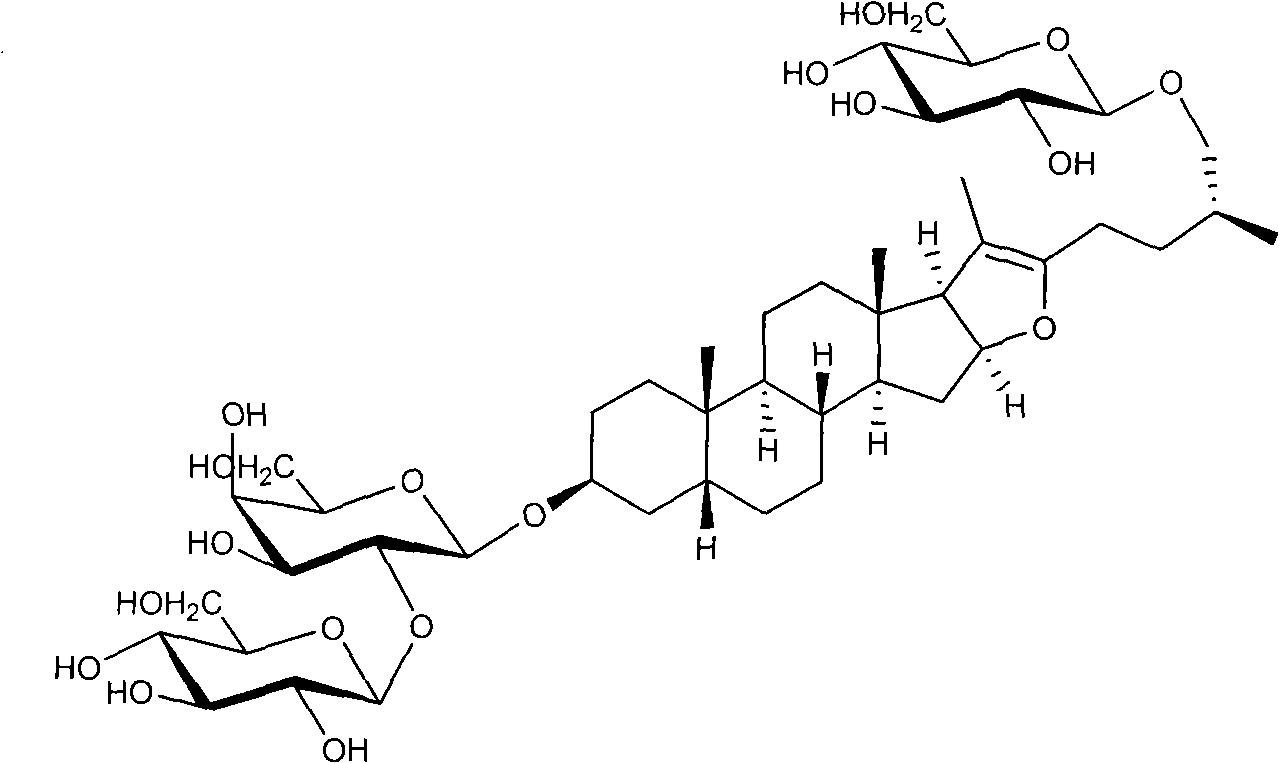
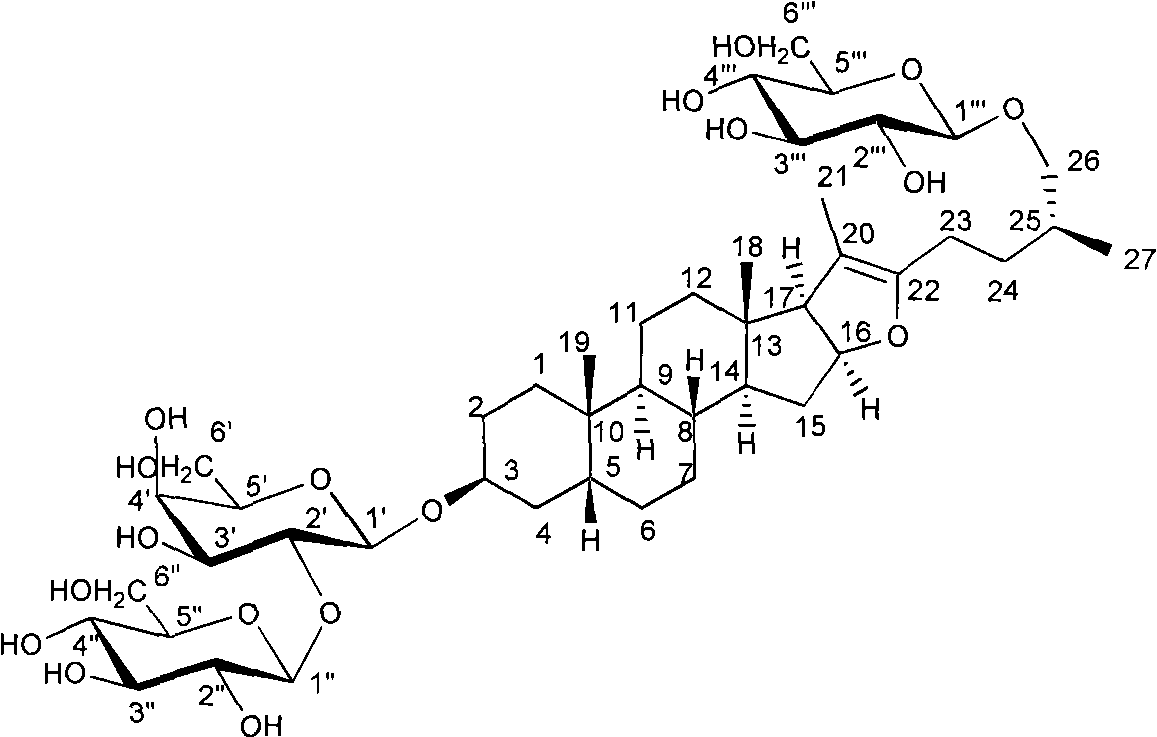
Method for preparing timosaponin BIII and uses thereof
A technology of timosaponin and mother saponin, which is applied in the field of preparation of plant extracts, can solve problems such as not being able to meet industrial applications, and achieve good anti-diabetic activity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Extraction and separation method one of timosaponin BIII
[0035] Take 5.0 kg of Anemarrhena medicinal material, degrease by gasoline percolation, and dry. Reflux extraction with 25 liters of 85% ethanol three times for 2 hours each time, filter, and combine the filtrates. Recover the solvent under reduced pressure, concentrate until it has no alcohol smell, and obtain 1 kg of mother extract.
[0036] Suspend the extract with 6 liters of water, and extract four times with 4 liters of n-butanol saturated with water. The n-butanol layer was recovered and dried to obtain 135 grams of extract. The sample was mixed with methanol for silica gel column (2700 g, 160-200 mesh) chromatography. First elute with chloroform until the color is light; then elute with chloroform-n-butanol (1:1). TLC detection, the developing solvent is n-butanol-ethyl acetate-water (4:1:5) upper layer. Using 10% phosphomolybdic acid ethanol at 110oC as the developer, the fractions co...
Embodiment 2
[0041] Embodiment 2: Extraction and separation method two of timosaponin BIII
[0042] Take 5.0 kg of Anemarrhena medicinal material, degrease by petroleum ether percolation, and dry. Reflux extraction with 40 liters of methanol three times for 2 hours each time, filter, and combine the filtrates. The solvent was recovered under reduced pressure, concentrated until there was no alcohol smell, and 1.2 kg of the mother extract was obtained.
[0043] Suspend the extract with 10 liters of water, and extract four times with 10 liters of n-pentanol saturated with water. The n-pentanol layer was recovered and dried to obtain 150 grams of extract. The sample was mixed with methanol for silica gel column (2700 g, 160-200 mesh) chromatography. First eluted with chloroform until the color became light; then eluted with chloroform-n-butanol (5:1). TLC detection, the developing solvent is n-butanol-ethyl acetate-water (4:1:5) upper layer. Using 10% ethanol phosphomolybdic acid as the ...
Embodiment 3
[0045] Embodiment 3: In vitro inhibition of α-glucosidase activity test
[0046] a) Reagents and instruments:
[0047] α-glucosidase (EC 232-604-7), purchased from Sigma Company, USA,
[0048] pNPG (4 nitrophenyl-α-D-glucopyranoside) (EC 223-189-3), purchased from Sigma
[0049] Anhydrous sodium carbonate, phosphate, etc., are analytically pure.
[0050] Microplate reader: BIO-TECK Instruments, produced in the United States.
[0051] b) Reagent preparation
[0052] Phosphate buffer (67mM, PH6.8): Weigh an appropriate amount of K 2 HPO 4 ·3H 2 O solution, adjusted to pH 6.8 with phosphoric acid, stored at 4°C until use.
[0053] Enzyme solution: Weigh an appropriate amount of α-glucosidase freeze-dried powder and dilute it with 67mM, pH 6.8 phosphate buffer solution at a concentration of 0.5mg / ml, aliquot 0.5ml into one tube, and freeze at -20°C.
[0054] Substrate PNPG: Prepare 29mM α-PNPG with phosphate buffer (67mM, pH 6.8), aliquot and store at -20°C.
[0055] Reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com