Method for synthesizing cis-lycopene isomer from full trans lycopene with photochemistry isomerization reaction
A technology of cis-lycopene and photochemical reaction, which is applied in the field of synthetic photochemistry, can solve problems such as difficult implementation, and achieve mild reaction conditions and environment-friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
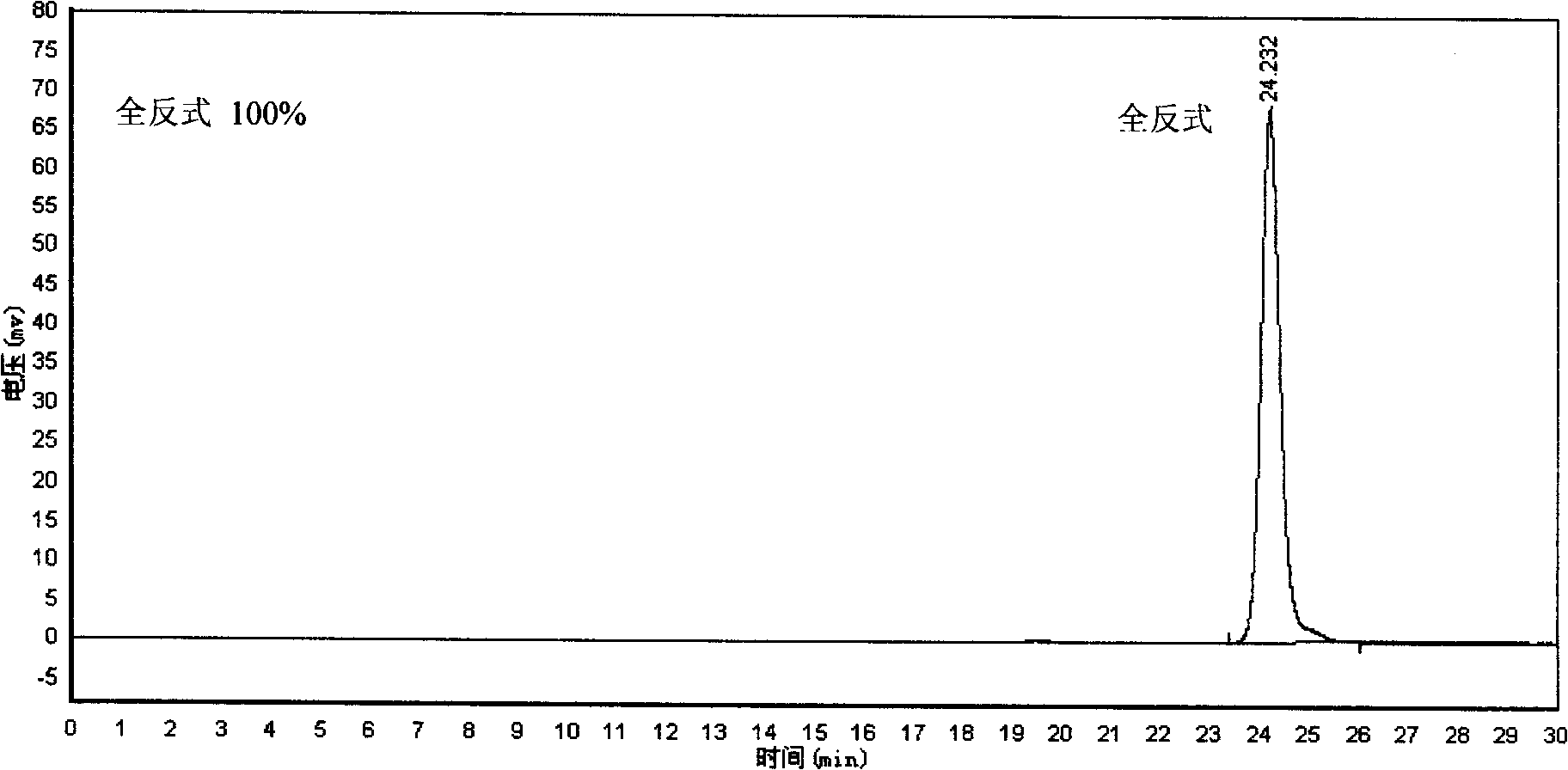
[0021] In a 30 ml glass test tube, 2 mg of all-trans lycopene was dissolved in 20 g of petroleum ether (60-90° C.) at a concentration of 0.01 wt%. Seal the mouth with a rubber stopper, and insert two injection needles on the rubber stopper. One is longer for nitrogen to pass into the photochemical reaction liquid, and the other is shorter for air and nitrogen mixed gas out of the photochemical reaction liquid surface. Adjusting the nitrogen flow rate does not cause the reaction liquid to overflow and make the bubbles uniform, on the one hand to protect the lycopene, on the other hand, it can also stir the photochemical reaction liquid to make the reaction uniform. Place the test tube 3 mm in front of the glass cold trap equipped with a 450-watt high-pressure mercury lamp (product of Beijing Electric Light Source Research Institute), and insert the borosilicate glass with a wavelength shorter than 283 nm between the test tube and the cold trap. Type filter. The reaction device is p...
Embodiment 2
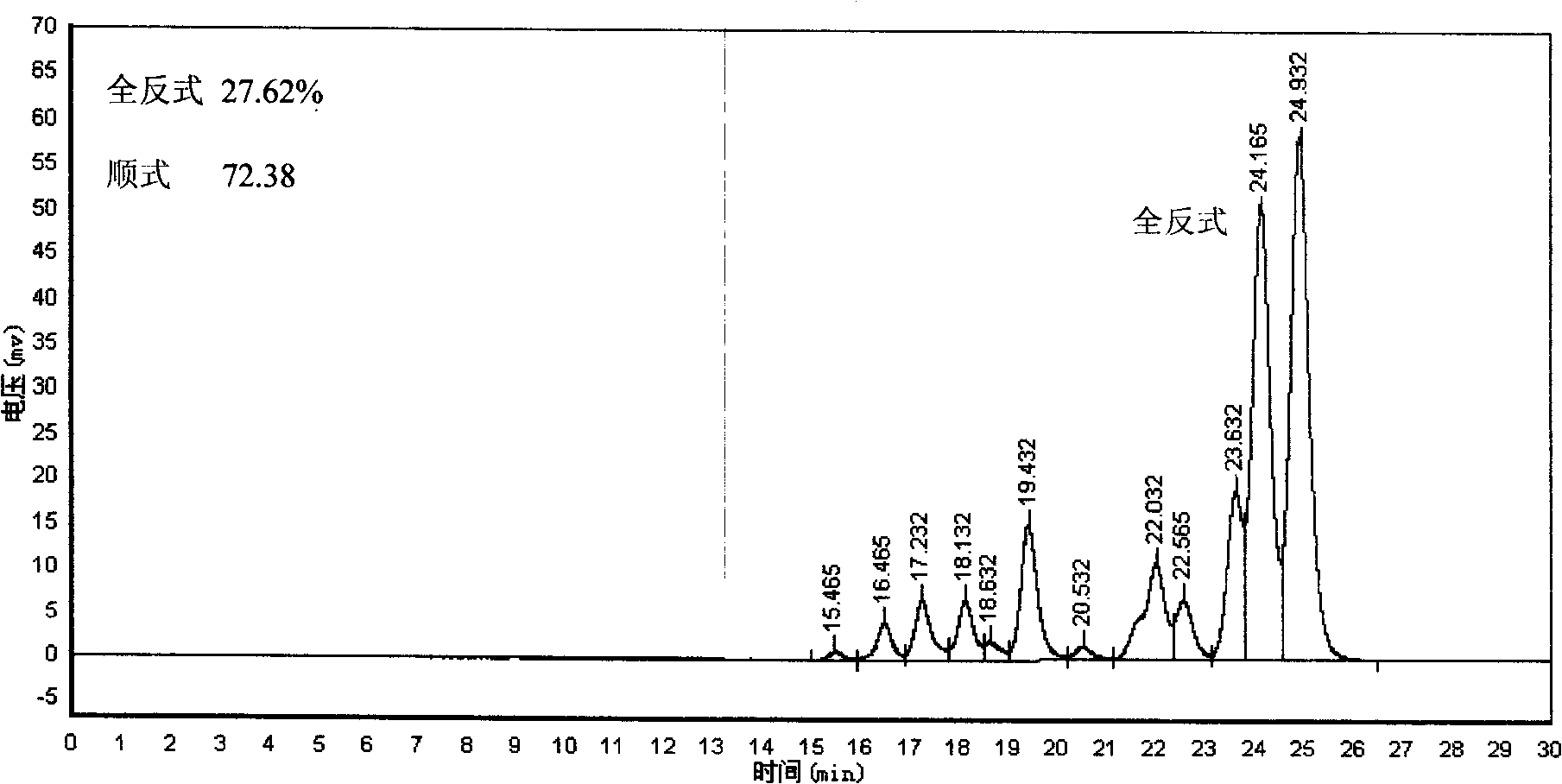
[0023] In a 30 ml glass test tube, 200 mg of all-trans lycopene was dissolved in 20 g of tetrahydrofuran with a concentration of 1 wt%. Seal the mouth with a rubber stopper, insert two injection needles into the rubber stopper, one longer for nitrogen to pass into the photochemical reaction liquid, and the other shorter for gas to be discharged outside the surface of the photochemical reaction liquid. Adjusting the nitrogen flow rate does not cause the reaction liquid to overflow and make the bubbles uniform, on the one hand to protect the lycopene, on the other hand, it can also stir the photochemical reaction liquid to make the reaction uniform. Place the test tube 3 mm in front of the glass cold trap equipped with a 450-watt high-pressure mercury lamp (product of Beijing Electric Light Source Research Institute), and insert the borosilicate glass with a wavelength shorter than 283 nm between the test tube and the cold trap. Type filter. The reaction device was placed in an opaq...
Embodiment 3
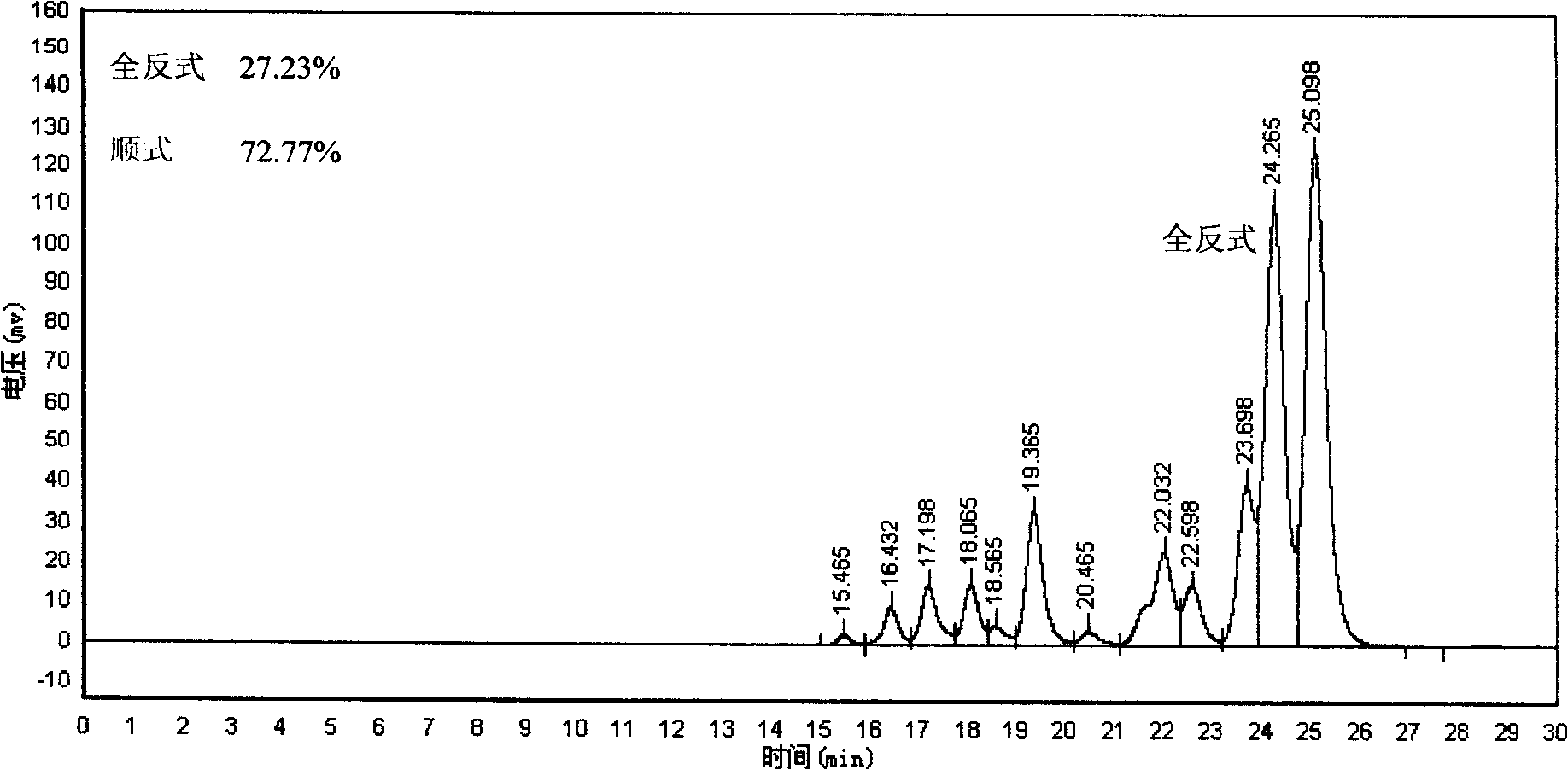
[0025] In a 30 ml glass test tube, 3 mg of all-trans lycopene was dissolved in 20 g of n-hexane at a concentration of 0.015 wt%. Seal the mouth with a rubber stopper, insert two injection needles into the rubber stopper, one longer for nitrogen to pass into the photochemical reaction liquid, and the other shorter for gas to be discharged outside the surface of the photochemical reaction liquid. Adjusting the nitrogen flow rate does not cause the reaction liquid to overflow and make the bubbles uniform, on the one hand to protect the lycopene, on the other hand, it can also stir the photochemical reaction liquid to make the reaction uniform. Place the test tube 3 mm in front of the glass cold trap equipped with a 450-watt high-pressure mercury lamp (product of Beijing Electric Light Source Research Institute), insert a cut-off filter with a wavelength shorter than 450 nanometers between the test tube and the cold trap sheet. The reaction device was placed in an opaque fume hood, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com