Method for separating benzoxazole oxazinone glycoside compounds from acanthus
A compound and a separation technology are applied in the field of separating benzoxazinone glycoside compounds from rat scorpion, and achieve the effects of less solvent consumption, organic solvent saving and fast separation speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
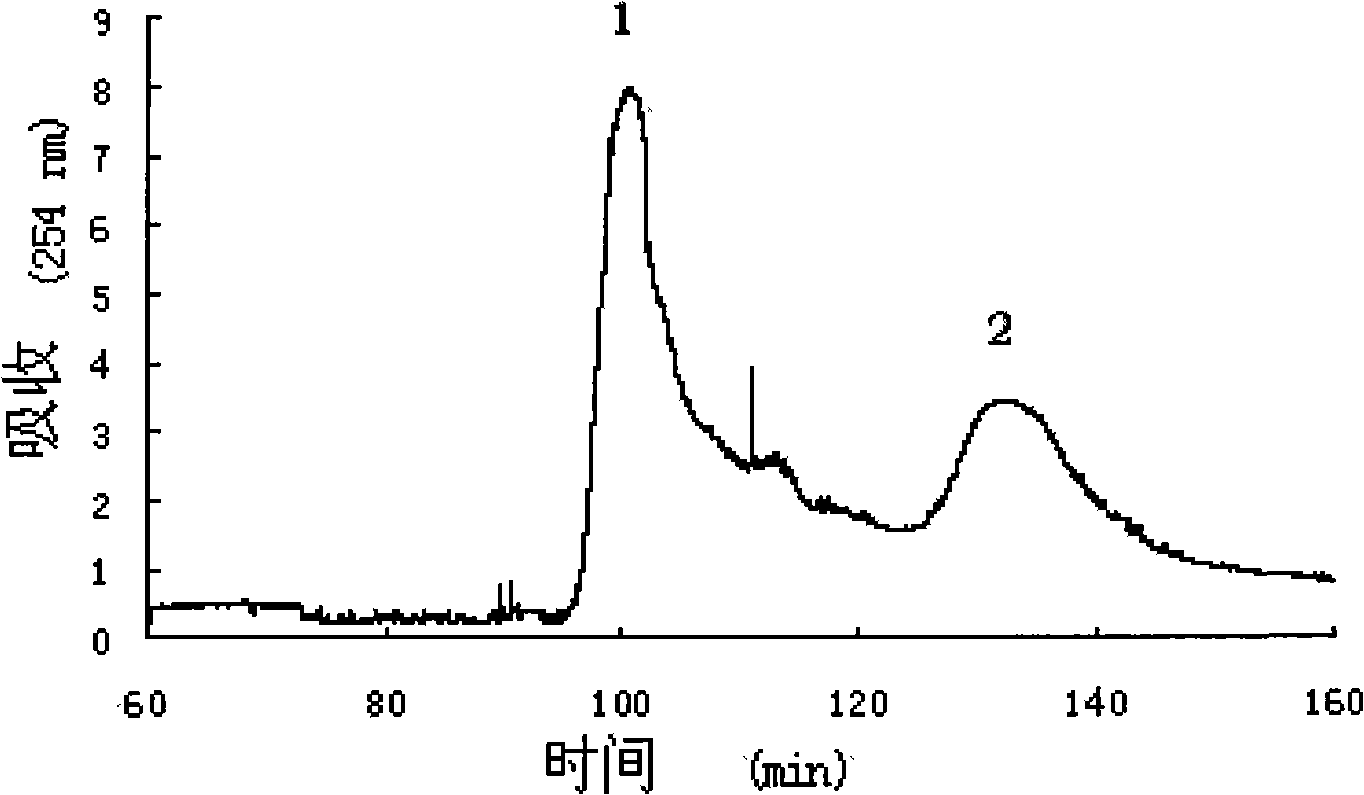
[0019] Dry 1kg of the aboveground part of rat botanicals and pulverize, extract 3 times with volume fraction of 95% ethanol (5000mL) at room temperature, evaporate the extract and suspend it in water, add petroleum ether to extract, separate the organic phase after layering (repeat 3 times) ), add ethyl acetate to the water phase for extraction, after layering, separate the organic phase (repeat 3 times), add n-butanol to the water phase for extraction, separate the organic phase (repeat 3 times), and distill the organic phase under vacuum After the solvent is removed, 40g of the n-butanol extract is obtained and stored at 4°C for later use. The HPLC analysis chromatogram of the n-butanol extract is shown in Figure 4 (1).
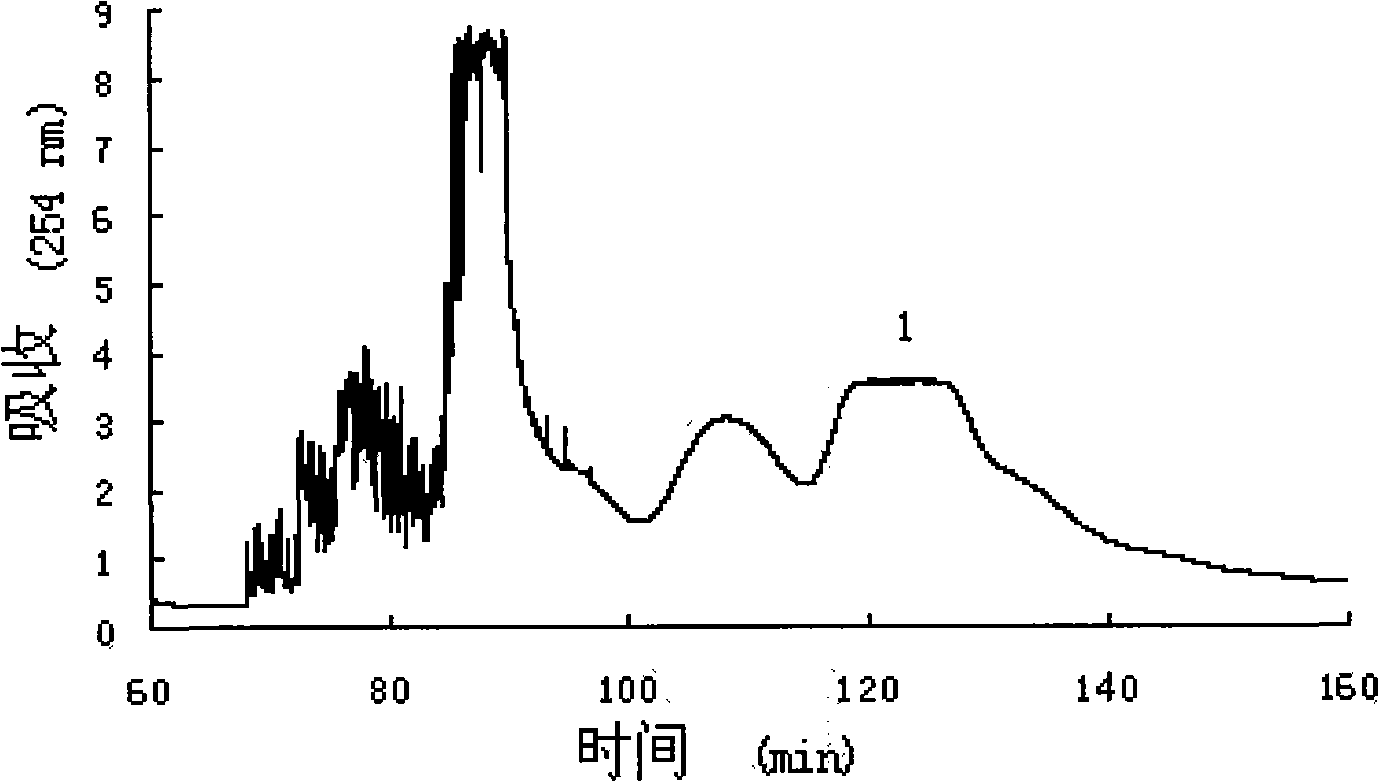
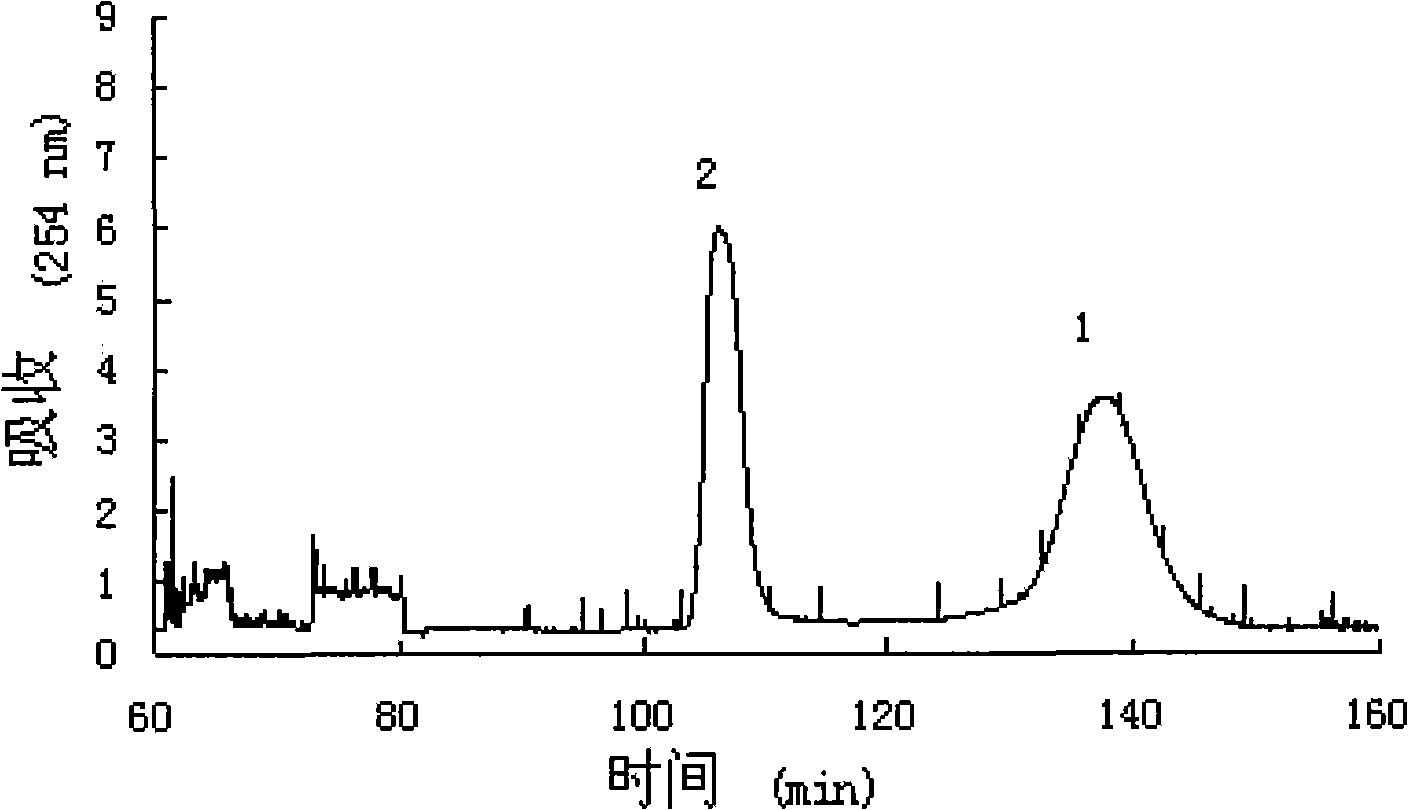
[0020] Ethyl acetate, n-butanol and 0.5% ammonia are mixed in a volume ratio of 2:3:5 to obtain a solvent system of 1000 mL. After mixing, the solvent is fully balanced at room temperature, and the two phases are separated before use. A sample of 100 mg of n-bu...
Embodiment 2
[0027] Dry 1kg of the aboveground part of rat botanicals and pulverize, extract 3 times with volume fraction of 95% ethanol (5000mL) at room temperature, evaporate the extract and suspend it in water, add petroleum ether to extract, separate the organic phase after layering (repeat 3 times) ), add ethyl acetate to the water phase for extraction, after layering, separate the organic phase (repeat 3 times), add n-butanol to the water phase for extraction, separate the organic phase (repeat 3 times), and distill the organic phase under vacuum After the solvent is removed, 40g of the n-butanol extract is obtained and stored at 4°C for later use. The HPLC analysis chart of the n-butanol extract is shown in Figure 4 (1).
[0028] The first step: mixing ethyl acetate, n-butanol and water in a volume ratio of 2:3:5 to obtain a solvent system of 10000 mL, the solvent is fully balanced at room temperature after mixing, and the two phases are separated before use. Take a sample of 1600 mg of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com