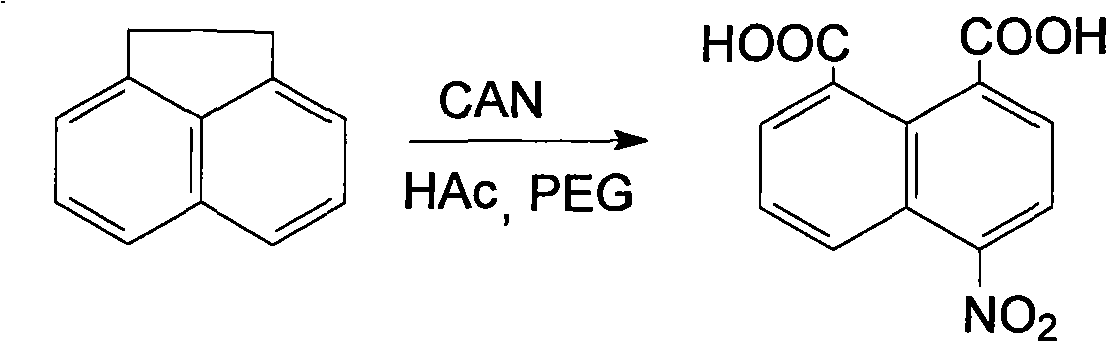Preparation of 4-nitro-1,8-naphthalic acid
A technology of naphthalene dicarboxylic acid and nitro, which is applied in the field of compound preparation, can solve problems such as environmental pollution and equipment corrosion, and achieve the effects of shortening reaction time, simple operation, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Add 1.542g (10mmol) acenaphthene, 40mL90% acetic acid (volume fraction), 0.223g (0.56mmol) polyethylene glycol-400, 13.710g (25mmol) cerium ammonium nitrate in the Erlenmeyer flask, install a reflux device, and heat to 30°C, reacted for 2.0 hours, wherein, TLC traced, the developer was acetone-petroleum ether (volume ratio 1:5), the reaction product was poured into ice water, a yellow precipitate was precipitated, filtered by suction, and the solid was washed 2-3 times with water. 30 mL each time, 3.901 g of crude product was obtained, which was recrystallized with 15 mL of 95% ethanol, and dried to obtain 4-nitro-1,8-naphthalene dicarboxylic acid. Weight: 2.060g, yield: 78.9%, mp: 151°C-153°C, literature value (140°C-150°C decomposition), IR: Vmax (KBr tablet, cm -1 ), 3127 (-OH), 1623 (C=O), 1595, 1508, 1400 (naphthalene ring skeleton vibration), 1508, 1325 (-NO 2 ), 818, 777 (=C-H), MS: m / z (M+H) + 260.9, 1 HNMR (DMSO-d 6 )δ: 8.60(s, 1H), 8.58(s, 1H), 7.48(d, 1H)...
Embodiment 2
[0014] Add 154.2g (1mol) acenaphthene, 0.5L90% acetic acid (volume fraction) in the reaction flask, 282.1g (0.705mol) polyethylene glycol-400, 1644.0g (3mol) cerium ammonium nitrate, heat to 50 ℃, react 1.6h, wherein TLC tracked, the developer was acetone-petroleum ether (volume ratio 1:5), the reaction product was poured into ice water to obtain a yellow precipitate, filtered by suction, and the solid was washed 2-3 times with 30mL each time to obtain crude The product was 388.4g. The crude product was recrystallized with 1L of 95% ethanol and dried to obtain 4-nitro-1,8-naphthalene dicarboxylic acid. Weight: 203.8g, yield: 78.1%, mp: 151°C-152.7°C, literature value (decomposition at 140°C-150°C), IR: Vmax (KBr tablet, cm -1 ), 3125 (-OH), 1628 (C=O), 1597, 1510, 1400 (naphthalene ring skeleton vibration), 1510, 1327 (-NO 2 ), 820, 779 (=C-H), MS: m / z (M+H) + 260.5, 1 HNMR (DMSO-d 6 )δ: 8.61(s, 1H), 8.58(s, 1H), 7.48(d, 1H), 8.52(d, 1H), 7.47(d, 1H), 7.76(m, 1H) 7.36(d, 1...
Embodiment 3
[0016] Add 154.2g (1mol) acenaphthene, 0.5L90% acetic acid (volume fraction) in the reaction flask, 282.1g (0.705mol) polyethylene glycol-400, 1644.0g (3mol) cerium ammonium nitrate, heat to 70 ℃, react 1.5h, wherein TLC tracked, the developer was acetone-petroleum ether (volume ratio 1:5), the reaction product was poured into ice water to obtain a yellow precipitate, filtered by suction, and the solid was washed 2-3 times with 30 mL each time to obtain crude The product was 388.4g. The crude product was recrystallized with 1L of 95% ethanol and dried to obtain 4-nitro-1,8-naphthalene dicarboxylic acid. Weight: 203.8g, yield: 78.1%, mp: 151°C-152.7°C, literature value (decomposition at 140°C-150°C), IR: Vmax (KBr tablet, cm -1 ), 3125 (-OH), 1628 (C=O), 1597, 1510, 1400 (naphthalene ring skeleton vibration), 1510, 1327 (-NO 2 ), 820, 779 (=C-H), MS: m / z (M+H) + 260.5, 1 HNMR (DMSO-d 6 )δ: 8.61(s, 1H), 8.58(s, 1H), 7.48(d, 1H), 8.52(d, 1H), 7.47(d, 1H), 7.76(m, 1H) 7.36(d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com