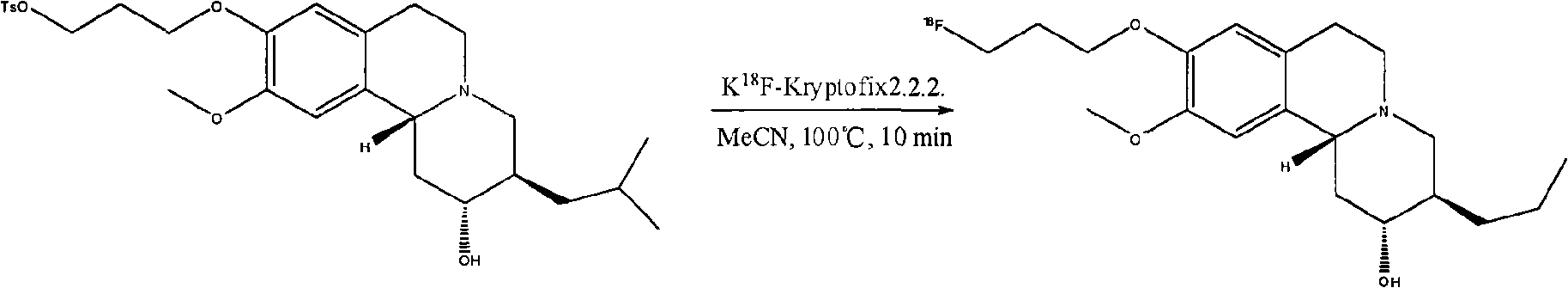Device and process capable of simultaneously preparing three different <18>F radiopharmaceuticals
A 18F and drug synthesis technology, applied in the field of imaging agent devices, can solve the problems of waste, high construction costs, and increased investment in PET centers, so as to achieve full and reasonable utilization and reduce investment costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
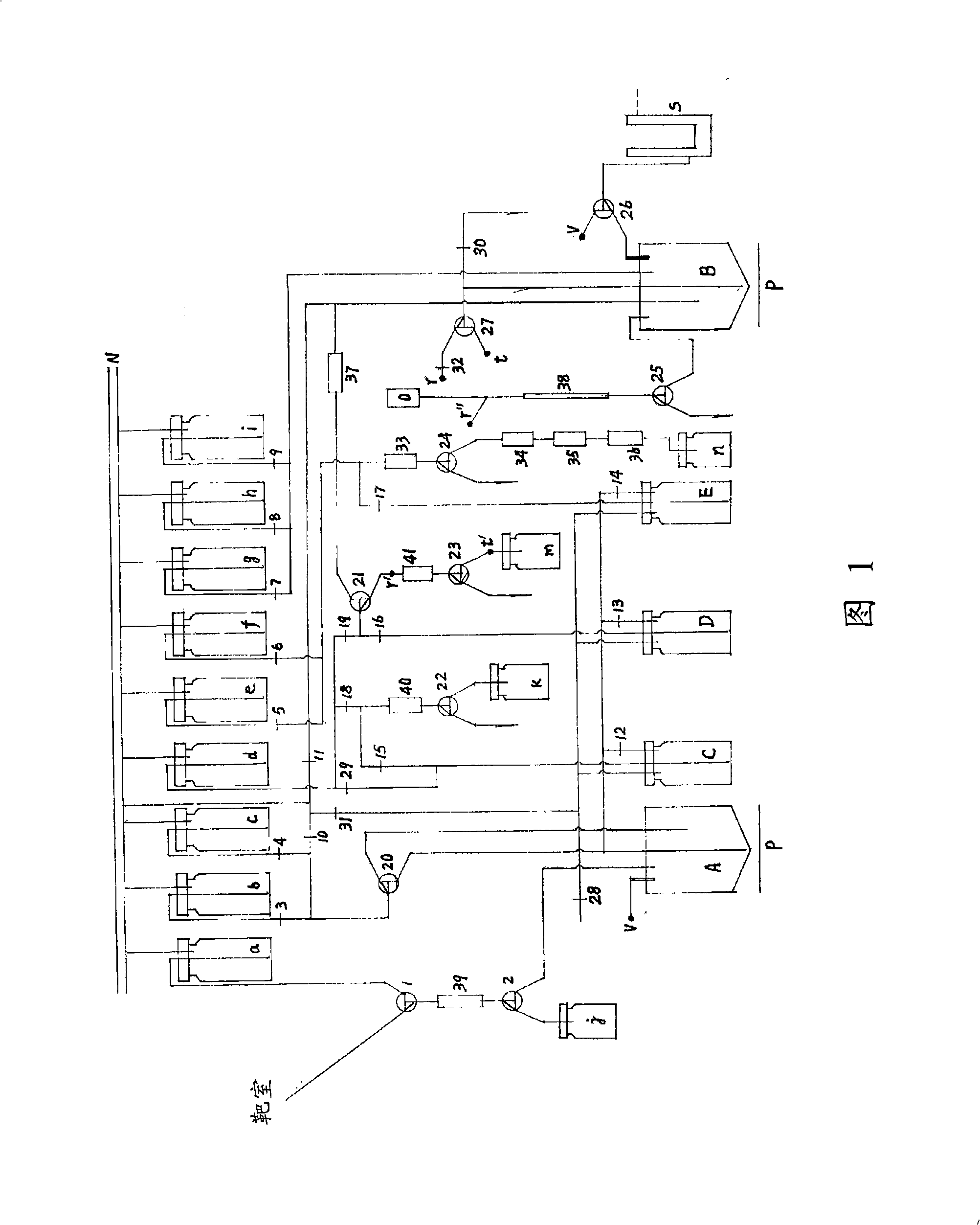
[0056] Embodiment 1, synthesized with the BNUF-A3 device shown in Figure 1 18 F-FET, 18 F-AV133 and 18 F-FDG
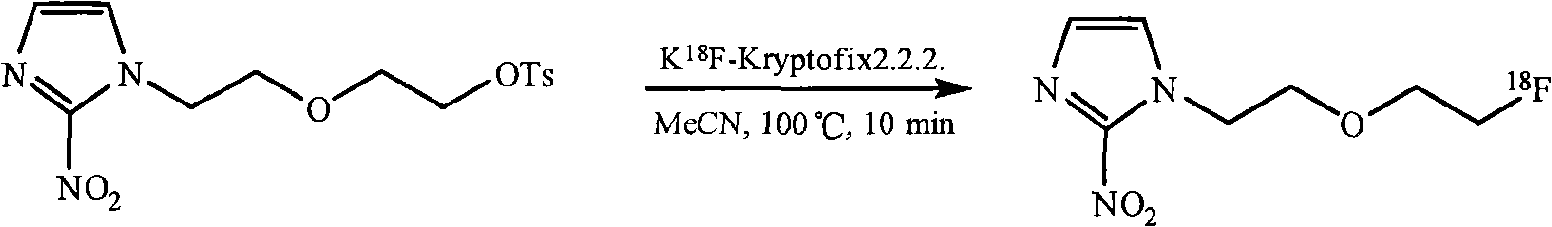
[0057] 1-(2-(2-fluoro[ 18 F] on behalf of ethoxy) ethyl-2-nitro-1-H-imidazole ( 18 F-FET) is a hypoxic imaging agent. Through 2-(2-(2-nitro-1 H-imidazole) ethoxy) ethyl p-toluenesulfonate and K 18 The acetonitrile solution of F-K2.2.2 was reacted at 100°C for 10 minutes to 18 F is substituted for p-toluenesulfonate to give 18 F-FETs. 18 The reaction formula of F-FET:
[0058]
[0059] 2-(2-(2-nitro-1H-imidazole)ethoxy)ethyl 1-(2-(2-fluoro[ 18 F] on behalf of ethoxy) ethyl -
[0060] p-Toluenesulfonate 2-nitro-1-H-imidazole
[0061] 18 F-AV133 is a molecular probe for early diagnosis of Parkinson's disease (PD). Similarly, 18 F-AV133 interacts with K through its precursor 18 The acetonitrile solution of F-K2.2.2 was reacted at 100°C for 10 minutes to make 18 Made by substituting p-toluenesulfonate at the end of the propoxy group.
[0062] 18 The re...
Embodiment 2
[0088] Embodiment 2, synthesize with BNUF-A3 two-step method shown in Fig. 1 18 F-FECNT
[0089] 18 The preparation of F-FECNT is through ethylene glycol 1,2-p-toluenesulfonate and K18 The acetonitrile solution of F-K2.2.2 was reacted at 100°C for 10 minutes to obtain the intermediate p-toluenesulfonic acid-2-fluoro[ 18 F] ethyl ester; then, p-toluenesulfonic acid-2-fluoro[ 18 F] Ethyl ester reacts with (2S,3S)-3-(p-chlorophenyl)-8-N-bicyclo[3,2,1]octane-2-acid methyl ester in DMF at 135°C 45 minutes to get it.
[0090] 18 F-FECNT synthetic route:
[0091]
[0092] 1,2-Ethylene p-toluenesulfonate p-toluenesulfonate-2-fluoro[ 18 F] ethyl ester
[0093]
[0094] (2S,3S)-3-(p-chlorophenyl)-8-N-bicyclic 18 F-FECNT
[0095] [3.2.1] Octyl-2-acid methyl ester
[0096] 1) Preparation:
[0097] Referring to Figure 1, connect thermal ports r-r" and t-t'; install a silica gel column at chromatographic column 37; inject 0.5 mL of acetonitrile solution of 1,2-di-p-toluenes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com