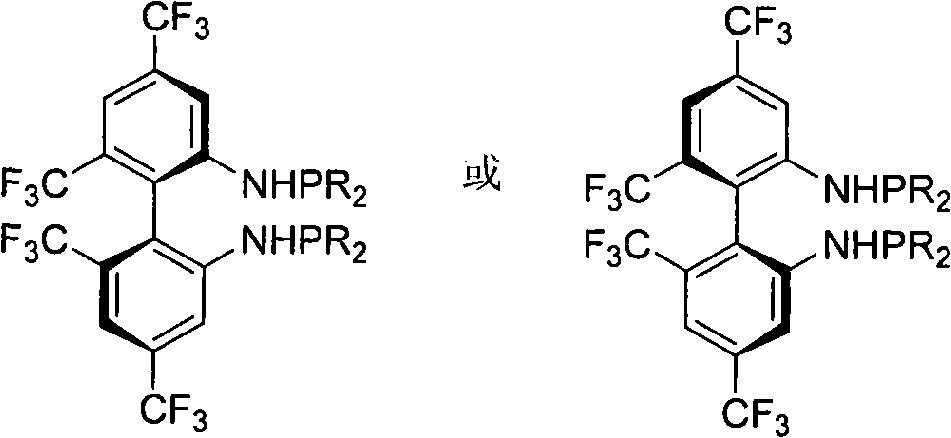
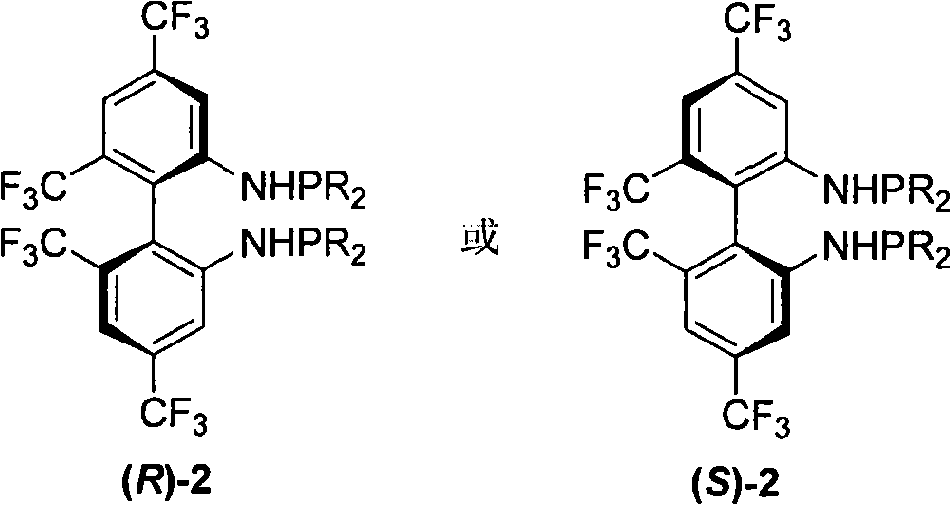
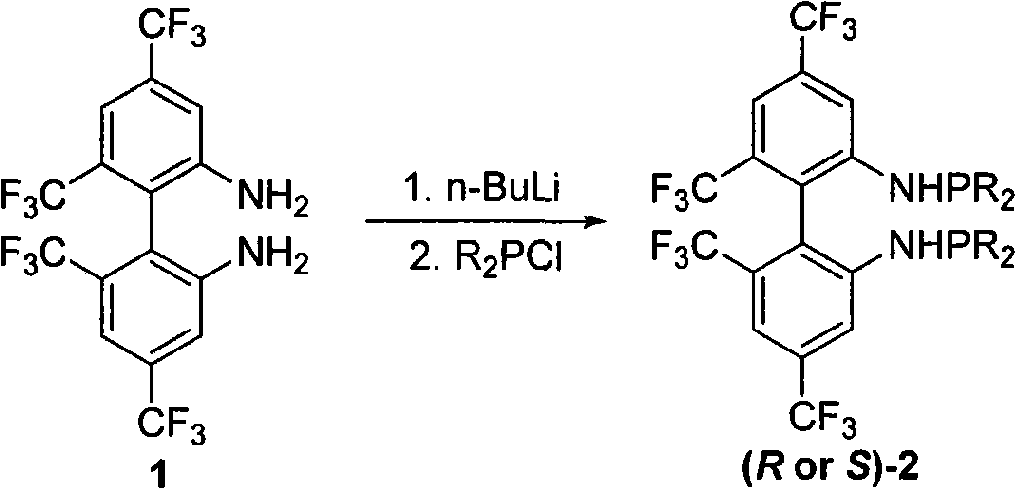Phosphoramidite type diphosphine ligand, preparation method and application thereof
A technology of bidentate phosphine ligands and phosphonamidites, which is applied in the field of phosphonamidite bidentate phosphine ligands and their preparation, and can solve problems such as difficulties in the synthesis of bisphosphine ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of 4,4',6,6'-tetrakis(trifluoromethyl)-N,N'-bis(diisopropylphosphino)-biphenyl-2,2'-diamine (R)-2a
[0026] Add 0.5 g of (R)-4,4',6,6'-tetrakis(trifluoromethyl)biphenyl-2,2'-diamine into a 50 mL Schlenk bottle, and add anhydrous and oxygen-free tetrahydrofuran under the protection of Ar gas 7.5 mL, at -40°C, add 2M n-BuLi (1.1 ml, 2.0 eq), react for 20-30 minutes, add diisopropylphosphine chloride (2.2 eq) with a syringe, and continue the reaction for 1 hour, naturally After warming up to room temperature and reacting for 24 hours, the solvent was spin-dried and subjected to basic alumina column chromatography (petroleum ether was used as eluent) to obtain 0.33 g of the product with a yield of 53.1%.
[0027] (R)-2a: mp 63-66°C; [α] 25 D =-188.8 (c 0.91, CHCl 3 ).IR (KBr) v 3371, 2967, 2938, 2897, 2870, 1620, 1584, 1508, 1441, 1398, 1386, 1286, 1265, 1170, 1138cm -1 ; 1 H NMR (CDCl 3 , TMS, 300MHz) δ0.78-0.99(m, 24H), 1.56-1.65(m, 4H), 3.68(d, J=8.7Hz,...
Embodiment 2
[0029] Preparation of 4,4',6,6'-tetrakis(trifluoromethyl)-N,N'-bis(diisopropylphosphino)-biphenyl-2,2'-diamine (R)-2a
[0030] Add 0.5 g of (R)-4,4',6,6'-tetrakis(trifluoromethyl)biphenyl-2,2'-diamine into a 50 mL Schlenk bottle, and add anhydrous and oxygen-free tetrahydrofuran under the protection of Ar gas Add 2M n-BuLi (1.1ml, 2.0eq) to 7.5mL at -20°C, react for 20-30 minutes, then add diisopropylsulfuric acid chloride (2.2eq) with a syringe, and continue to react for 1h. After warming up to room temperature and reacting for 24 hours, the solvent was spin-dried and subjected to basic alumina column chromatography (petroleum ether was used as eluent) to obtain 0.31 g of the product with a yield of 50.0%.
Embodiment 3
[0032] Preparation of 4,4',6,6'-tetrakis(trifluoromethyl)-N,N'-bis(diisopropylphosphino)-biphenyl-2,2'-diamine (R)-2a
[0033] Add 0.5 g of (R)-4,4',6,6'-tetrakis(trifluoromethyl)biphenyl-2,2'-diamine into a 50 mL Schlenk bottle, and add anhydrous and oxygen-free tetrahydrofuran under the protection of Ar gas 7.5mL, at 0℃, add 2M n-BuLi (1.1ml, 2.0eq), react for 20-30 minutes, add diisopropylphosphine chloride (2.2eq) with a syringe, continue to react for 1h, then raise the temperature naturally After reacting at room temperature for 24 hours, the solvent was spin-dried and subjected to basic alumina column chromatography (petroleum ether was used as eluent) to obtain 0.37 g of the product with a yield of 60.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com