Method of surface nano-crystallization of noble metal catalyst
A noble metal catalyst, nanotechnology, applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, chemical instruments and methods, etc., can solve the problems of fragility and limited application, and achieve high mechanical strength , high specific surface area, performance improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
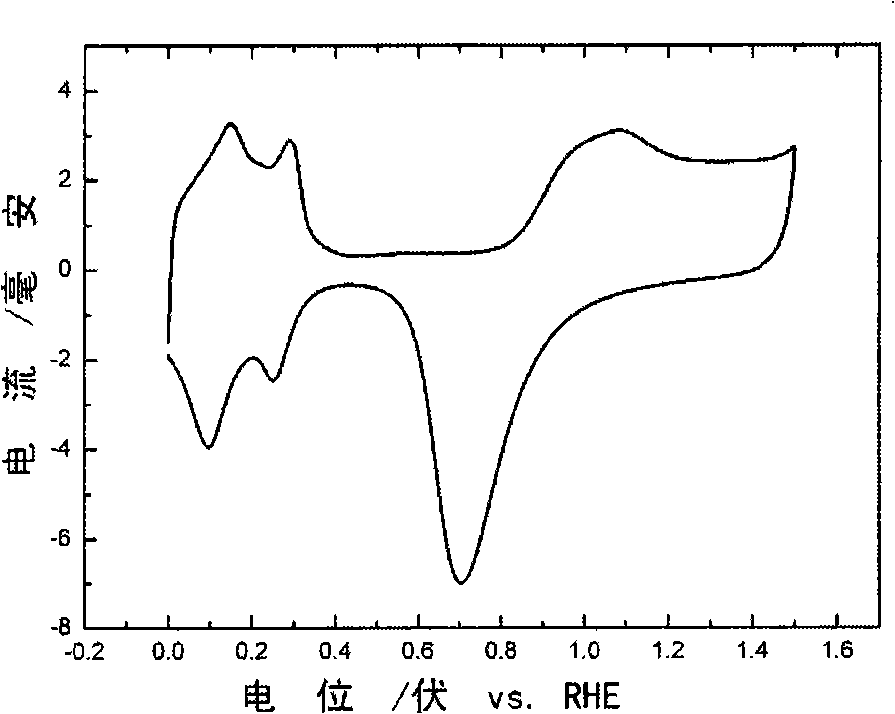
[0045] 1) Anneal a platinum wire with a length of 3 cm and a diameter of 0.1 mm at 500 ° C for 16 h, soak it in 68 wt.% nitric acid for 2 h, clean it with ultrapure water, and 2 SO 4 In the process, the reversible hydrogen electrode was used as the reference electrode, and the cyclic voltammetry curve was scanned between 0-1.5V.
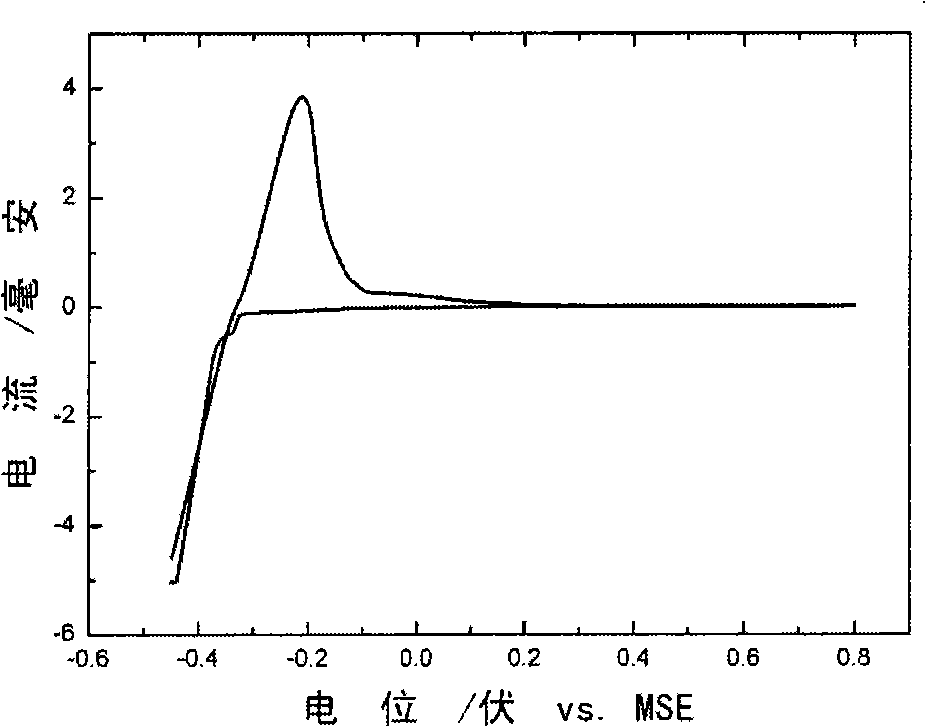
[0046] 2) CuSO at 0.1mol / L 4 In the solution, the mercurous mercurous sulfate electrode is used as the reference electrode, and the cyclic voltammetry curve is scanned between -0.45-0.8V (such as figure 1 ), and then deposited copper at a constant potential of -0.4V for 10000s.
[0047] 3) The copper-deposited platinum wire was annealed at 700° C. for 3 h under the protection of hydrogen.
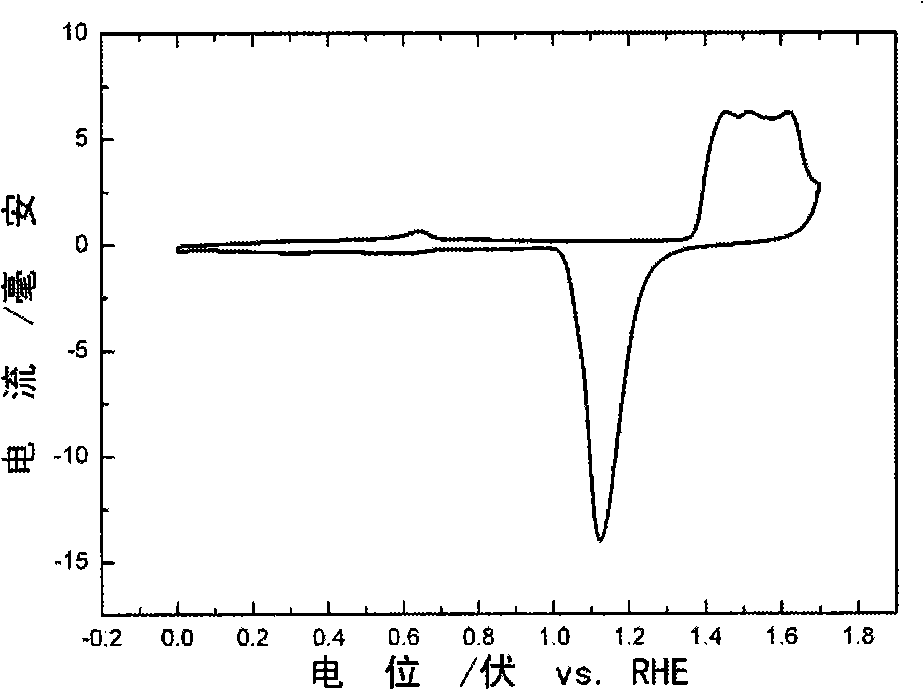
[0048] 4) The sample after annealing was heated at 0.5mol / L H 2 SO 4 In this method, the reversible hydrogen electrode was used as the reference electrode, and the electrolytic corrosion was performed at a potential of 1.3V for 10000s to prepare a platinum wi...
Embodiment 2
[0050] 1) Anneal a gold wire with a length of 3 cm and a diameter of 0.1 mm at 500 ° C for 16 h, soak it in 60 wt.% nitric acid for 2 h, clean it with ultrapure water, and 2 SO 4 In the process, the reversible hydrogen electrode was used as the reference electrode, and the cyclic voltammetry curve was scanned between 0-1.8V.
[0051] 2) CuSO at 0.1mol / L 4 In the solution, the mercury mercurous sulfate electrode was used as the reference electrode, and the cyclic voltammetry curve was scanned between -0.45-1.1V, and then copper was deposited at the constant potential of -0.45V for 10000s.
[0052] 3) The copper-deposited gold wire was annealed at 700° C. for 3 h under the protection of hydrogen.
[0053] 4) The sample after annealing was heated at 0.5mol / L H 2 SO 4 In this method, using a reversible hydrogen electrode as a reference electrode, electrolytically corroded for 10,000 s at a potential of 1.4V, a gold wire with a nanoporous surface was prepared. Its in 0.5mol / L ...
Embodiment 3
[0055] 1) Platinum mesh with a length of 1 cm and a width of 0.5 cm (such as Figure 8 shown) annealed at 500°C for 16h, soaked in 70wt.% nitric acid for 2h, cleaned with ultrapure water, and then heated at 0.5mol / LH 2 SO 4 In the process, the reversible hydrogen electrode was used as the reference electrode, and the cyclic voltammetry curve was scanned between 0-1.5V.
[0056] 2) CuSO at 0.1mol / L 4 In the solution, the mercurous mercurous sulfate electrode is used as the reference electrode, and the cyclic voltammetry curve is scanned between -0.45-0.8V, and then the copper is deposited at the constant potential of -0.45V for 22000s (such as Figure 9 shown).
[0057] 3) The copper-deposited platinum mesh was annealed at 700° C. for 3 h under the protection of hydrogen.
[0058] 4) The sample after annealing was heated at 0.5mol / L H 2 SO 4 In this method, the reversible hydrogen electrode was used as a reference electrode, and electrolytic corrosion was performed at a p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com