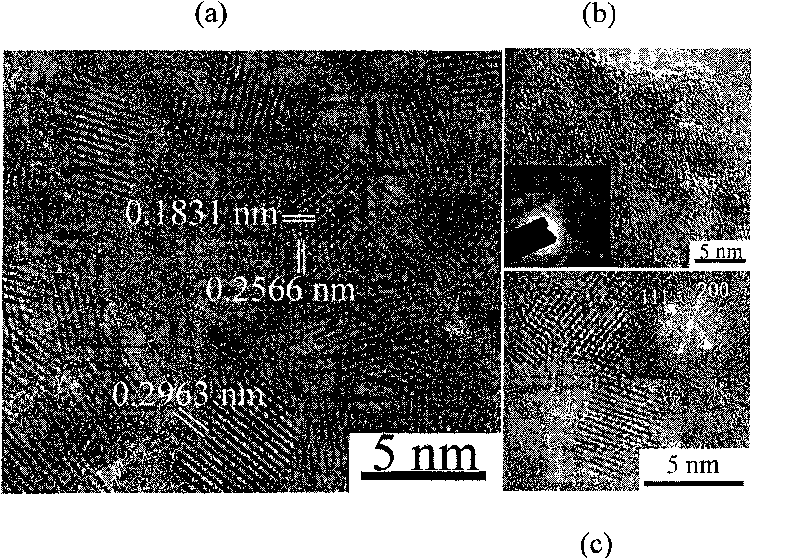
Method for preparing metal cation modified cubic phase zirconia nanometer particles
A metal cation, phase zirconia technology, applied in zirconia and other directions, can solve problems such as increasing costs, and achieve the effects of low equipment requirements, high yield and fewer types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 1.6g of sodium hydroxide into a 50ml beaker containing 30ml of benzyl alcohol, and stir for about 4 hours until the sodium hydroxide is dispersed in the benzyl alcohol, and the solution is a viscous transparent colloid; then add 1.611g (5mmol ) ZrOCl 2 ·8H 2Slowly add O to the above transparent colloid, stir until ZrOCl 2 ·8H 2 O was also dispersed in the colloid, and then 5ml of oleic acid was added dropwise to the above mixture, and the mixture was a yellow viscous solid at this time. After stirring vigorously for 3 hours, the reactant was transferred to a 50 ml stainless steel reaction kettle with a polytetrafluoroethylene liner, sealed well, and placed in a constant temperature oven at 160° C. for 24 hours. When the reaction kettle is naturally cooled to room temperature, take the white solid in the bottom layer of the kettle, put it in a 40ml centrifuge tube, add 30ml of pure water, centrifuge at a speed of 7000r / min for 5 minutes, wash repeatedly 7 to 8 tim...
Embodiment 2
[0029] Add 2.807g of potassium hydroxide to a 50ml beaker containing 30ml of benzyl alcohol, and stir for about 4 hours until the potassium hydroxide is dispersed in the benzyl alcohol, and the solution is a viscous transparent colloid; then 1.611g (5mmol ) ZrOCl 2 ·8H 2 Slowly add O to the above transparent colloid, stir until ZrOCl 2 ·8H 2 O was also dispersed in the colloid, and then 5ml of oleic acid was added dropwise to the above mixture, and the mixture was a yellow viscous solid at this time. After stirring vigorously for 3 hours, the reactant was transferred to a 50ml stainless steel reaction kettle with a polytetrafluoroethylene liner, sealed well, and placed in a constant temperature oven at 160°C for 24 hours to react. When the reaction kettle is naturally cooled to room temperature, take the white solid in the bottom layer of the kettle, put it in a 40ml centrifuge tube, add 30ml of pure water, centrifuge at a speed of 7000r / min for 5 minutes, wash repeatedly 7...
Embodiment 3
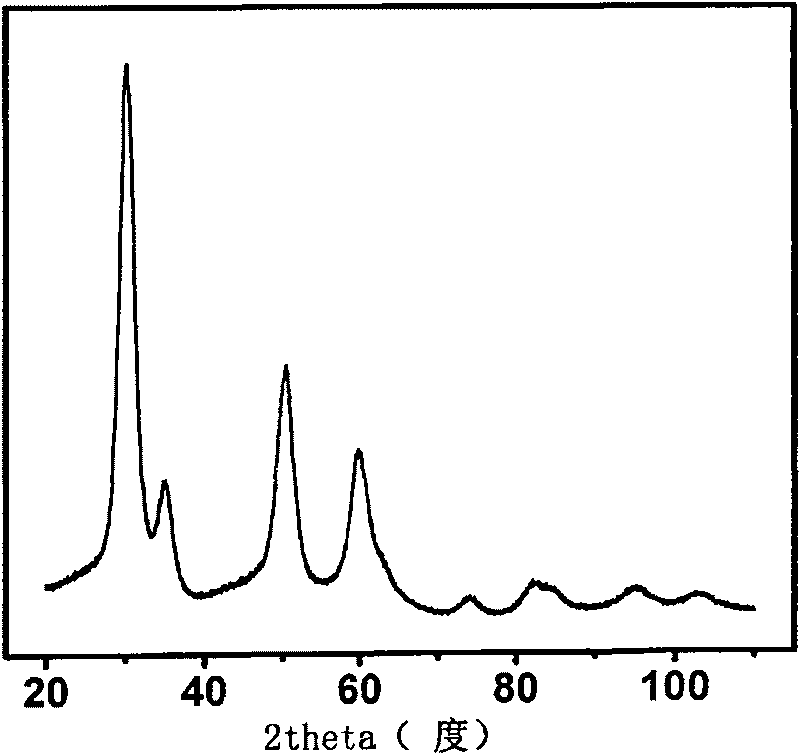
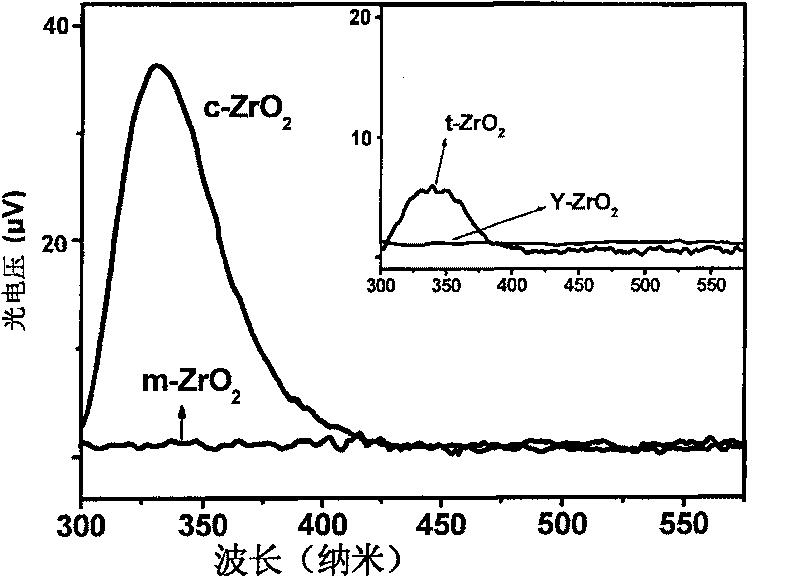
[0031] Disperse the sodium or potassium modified zirconia sample prepared in 100mg of Example 2, 3 into 20ml, 0.5M M n+ (M=Li, Cs, Mg, Ca, Sr, Ba, Al, Fe, Co) aqueous solution (the preparation of each aqueous solution uses the chloride salt or nitrate of the corresponding ion), after 12 hours of immersion After stirring, centrifuge and wash with deionized water, repeat 3 to 4 times until the corresponding cations are not detected in the cleaning solution, and dry naturally or in vacuum to obtain zirconia nanoparticles with cubic structure modified by other ions. from Figure 6 It can be concluded from the X-ray powder diffraction that the ion-exchanged zirconia material still has a cubic structure. Figure 7 and Figure 8 They are the surface photovoltage and surface photocurrent spectra of various cation-modified cubic zirconia, respectively. It can be seen from the figure that Al ion-modified zirconia materials have obvious advantages.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com