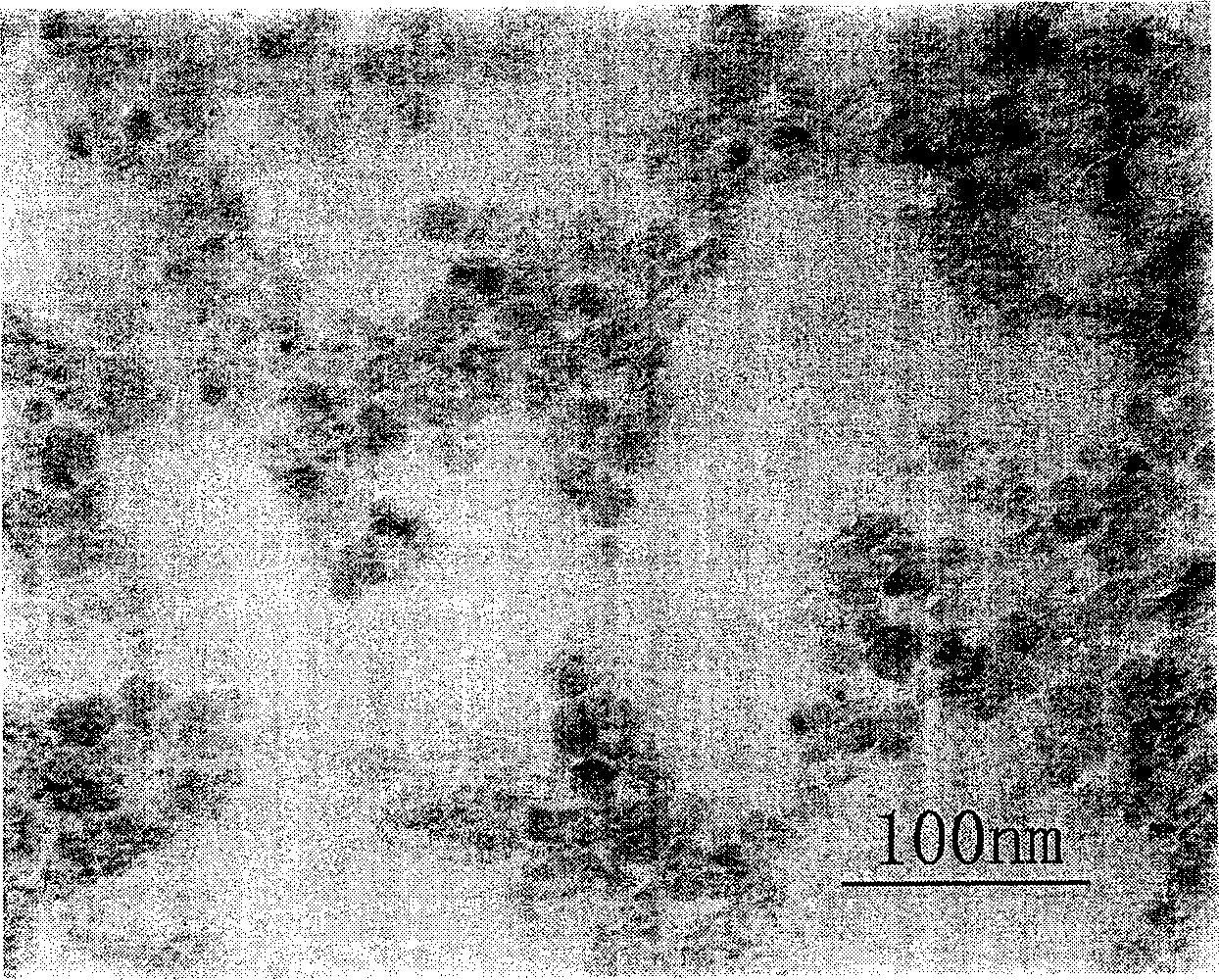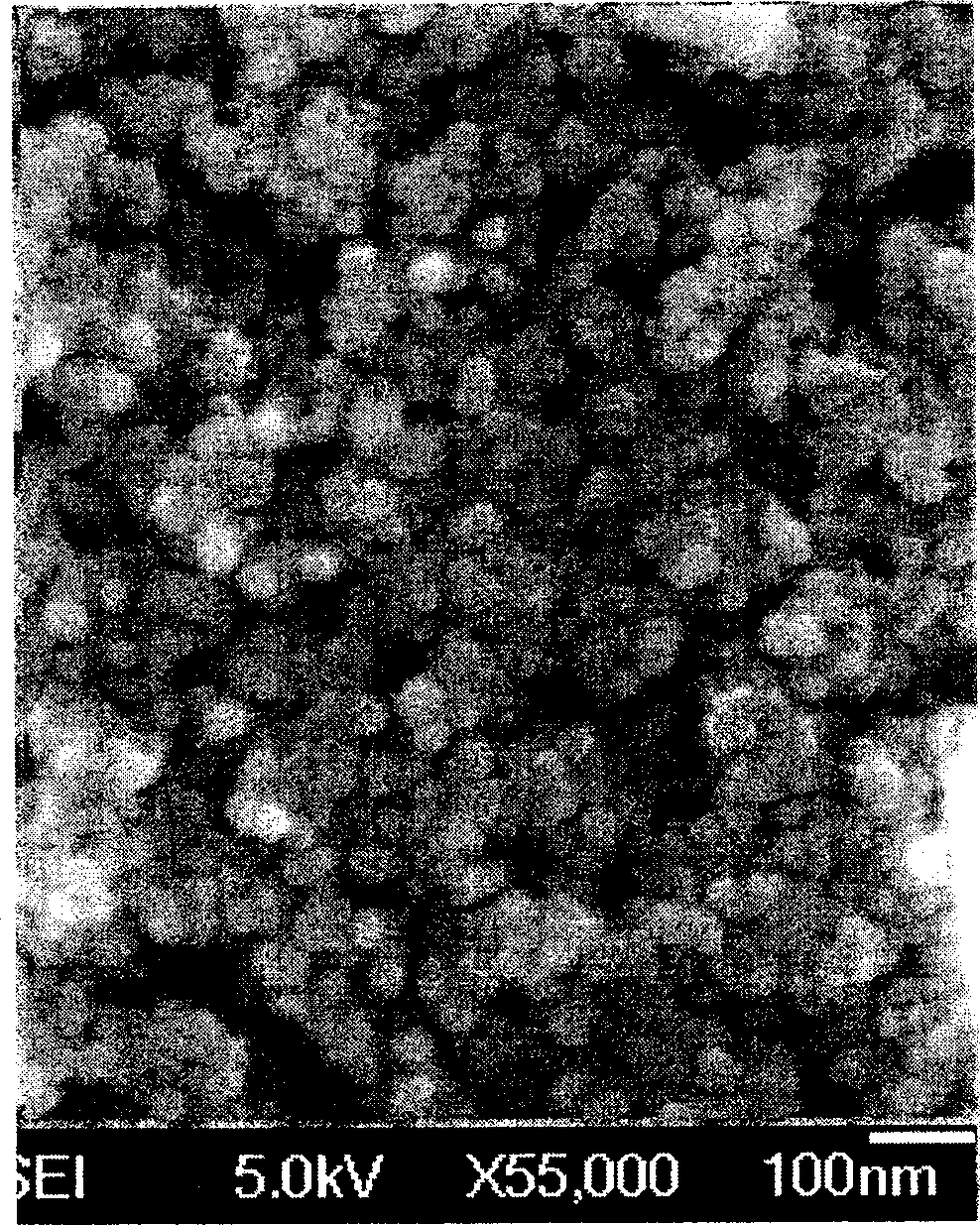Superparamagnetic Fe3O4 nanometer particle with synthetic polymer modification from one-step method
A technology for synthesizing polymers and nanoparticles, which is applied in the treatment of dyed polymer organic compounds and fibrous fillers, etc. It can solve the problems of difficult control of particle size, high viscosity of polymer sol, and non-coating of magnetic nanoparticles to achieve reaction The effects of different kinds of substances are easy to obtain, the method is simple and direct, and there are few kinds of reactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 2.5 g of acrylic acid and 1.38 g of ferrous chloride tetrahydrate into a reaction vessel filled with 50 ml of anaerobic distilled water, and slowly stir for 30 minutes under nitrogen protection. Then under the protection of nitrogen in the range of 85°C, stir vigorously at a constant temperature, and add 200 ml of a mixed solution containing 0.338 g of potassium peroxodisulfate and 1.8 g of sodium hydroxide dropwise to the reaction vessel within 4 hours. Then continue to react for 30 minutes while stopping heating and naturally cooling to room temperature. Centrifuge, wash with water, and centrifuge again to obtain a black precipitate, a light black powder dried in vacuum at room temperature, and redisperse the black precipitate in a mixed solution of ethanol and water to obtain a stable sol, which can be stored for a long time.
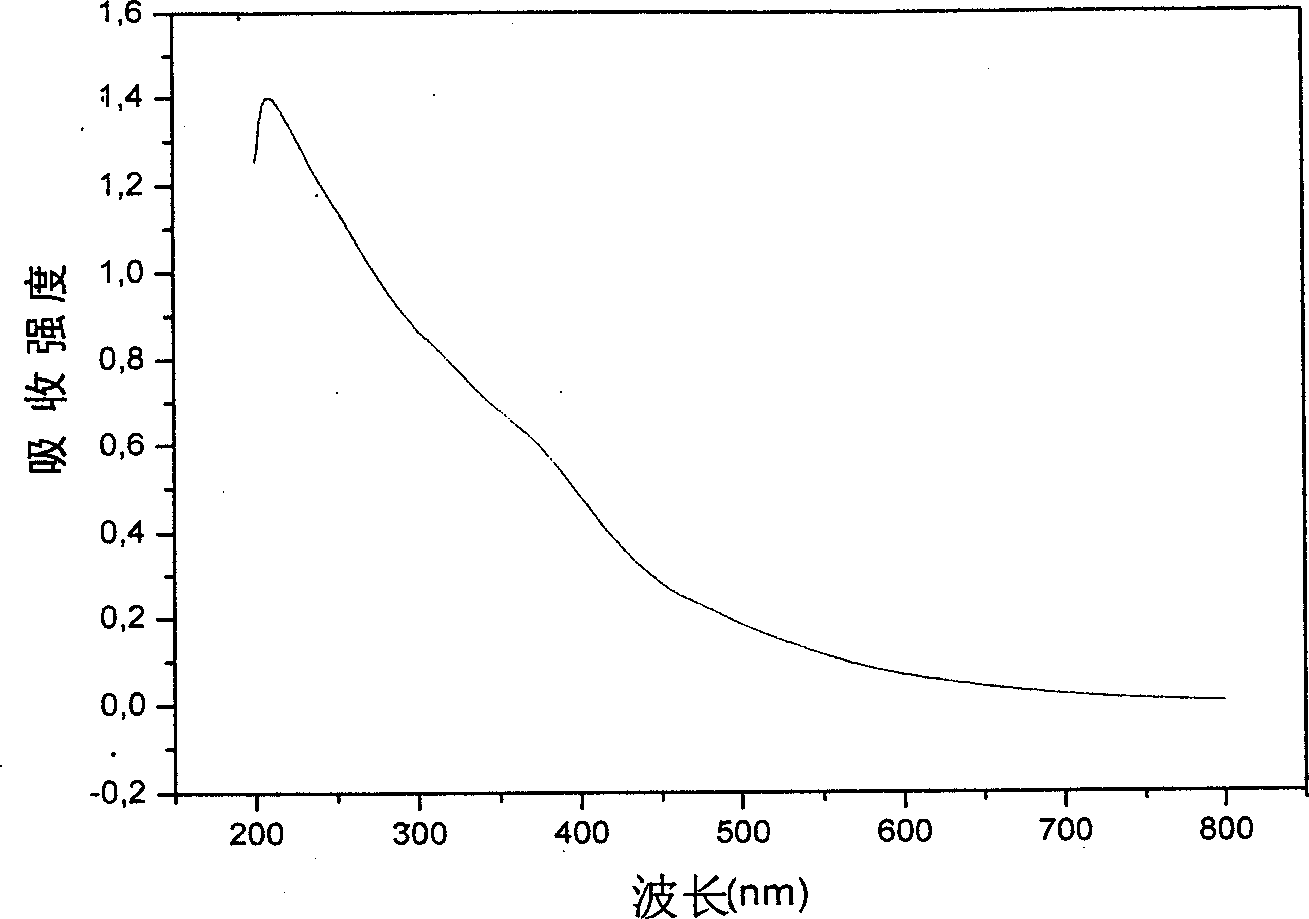
[0025] Some structures and properties of the prepared polymer-coated magnetic nanoparticles were characterized. TEM characterization of ...
Embodiment 2
[0027] Add 2.5 g of acrylic acid and 0.3452 g of ferrous chloride tetrahydrate into a reaction vessel filled with 50 ml of anaerobic distilled water, and slowly stir for 30 minutes under nitrogen protection. Then under the protection of nitrogen in the range of 85°C, stir vigorously at a constant temperature, and add 200 ml of a mixed solution containing 0.1688 g of potassium peroxodisulfate and 1.8 g of sodium hydroxide dropwise to the reaction vessel within 3 hours. Then continue to react for 30 minutes while stopping heating and naturally cooling to room temperature. Centrifuge, wash with water, and centrifuge again to obtain a black precipitate, a light black powder dried in vacuum at room temperature, and redisperse the black precipitate in a mixed solution of ethanol and water to obtain a stable sol, which can be stored for a long time, and the particle size ratio can be implemented The one prepared in Example 1 is smaller.
Embodiment 3
[0029] Add 5.0 g of acrylic acid and 0.6903 g of ferrous chloride tetrahydrate into a reaction vessel filled with 50 ml of anaerobic distilled water, and slowly stir for 30 minutes under nitrogen protection. Then, under the protection of nitrogen in the range of 85°C, stir vigorously at a constant temperature, and add 200 ml of a mixed solution containing 0.253 g of potassium peroxodisulfate and 2.63 g of sodium hydroxide dropwise to the reaction vessel within 4 hours. Then continue to react for 30 minutes while stopping heating and naturally cooling to room temperature. Centrifuge, wash with water, and centrifuge again to obtain a black precipitate, a light black powder dried in vacuum at room temperature, and redisperse the black precipitate in a mixed solution of ethanol and water to obtain a stable sol, which can be stored for a long time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com