Azo compound or salts thereof
A technology of azo compound and sulfamoyl group, applied in azo dyes, disazo dyes, monoazo dyes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] After adding 400 parts of water to 40.0 parts of p-aminobenzenesulfonic acid represented by formula (a-1), pH was adjusted to 7-8 with 30% sodium hydroxide aqueous solution under ice-cooling. The following operations were carried out under ice cooling. Add 19.1 parts of sodium nitrite and stir for 30 minutes. 72.2 parts of 35% hydrochloric acid were added a little at a time to form a brown solution and stirred for 2 hours. Dissolve 4.4 parts of sulfamic acid in 30 parts of water to form an aqueous solution, add the aqueous solution into the reaction solution, and stir to obtain a suspension containing diazonium salt.
[0062]
[0063] After adding 800 parts of water to 54.4 parts of 1-ethyl-3-carbamoyl-4-methyl-6-hydroxypyridin-2-one represented by formula (c-1), use 30% sodium hydroxide to The aqueous solution adjusts the pH to 8-9.
[0064]
[0065] The following operations were carried out under ice cooling. After stirring the above pyridone aqueous solutio...
Embodiment 2
[0072] Put 5 parts of azo compound (I-2), 50 parts of chloroform, and 2.1 parts of N,N-dimethylformamide into a flask equipped with a condenser tube and a stirring device, and keep the temperature below 20°C while stirring Add 6 parts of thionyl chloride dropwise. After completion of the dropwise addition, the temperature was raised to 50°C, the same temperature was maintained for 5 hours to allow the reaction to proceed, and then cooled to 20°C. A mixed solution of 4 parts of 3-isopropoxypropylamine and 14 parts of triethylamine was added dropwise while maintaining the temperature of the cooled reaction solution at 20° C. or lower while stirring. Then, stirring was carried out at the same temperature for 5 hours to allow the reaction to proceed. Next, the solvent in the obtained reaction mixture was evaporated with a rotary evaporator, and then a small amount of methanol was added, followed by vigorous stirring. This mixture was added to a liquid mixture of 29 parts of acet...
Embodiment 3
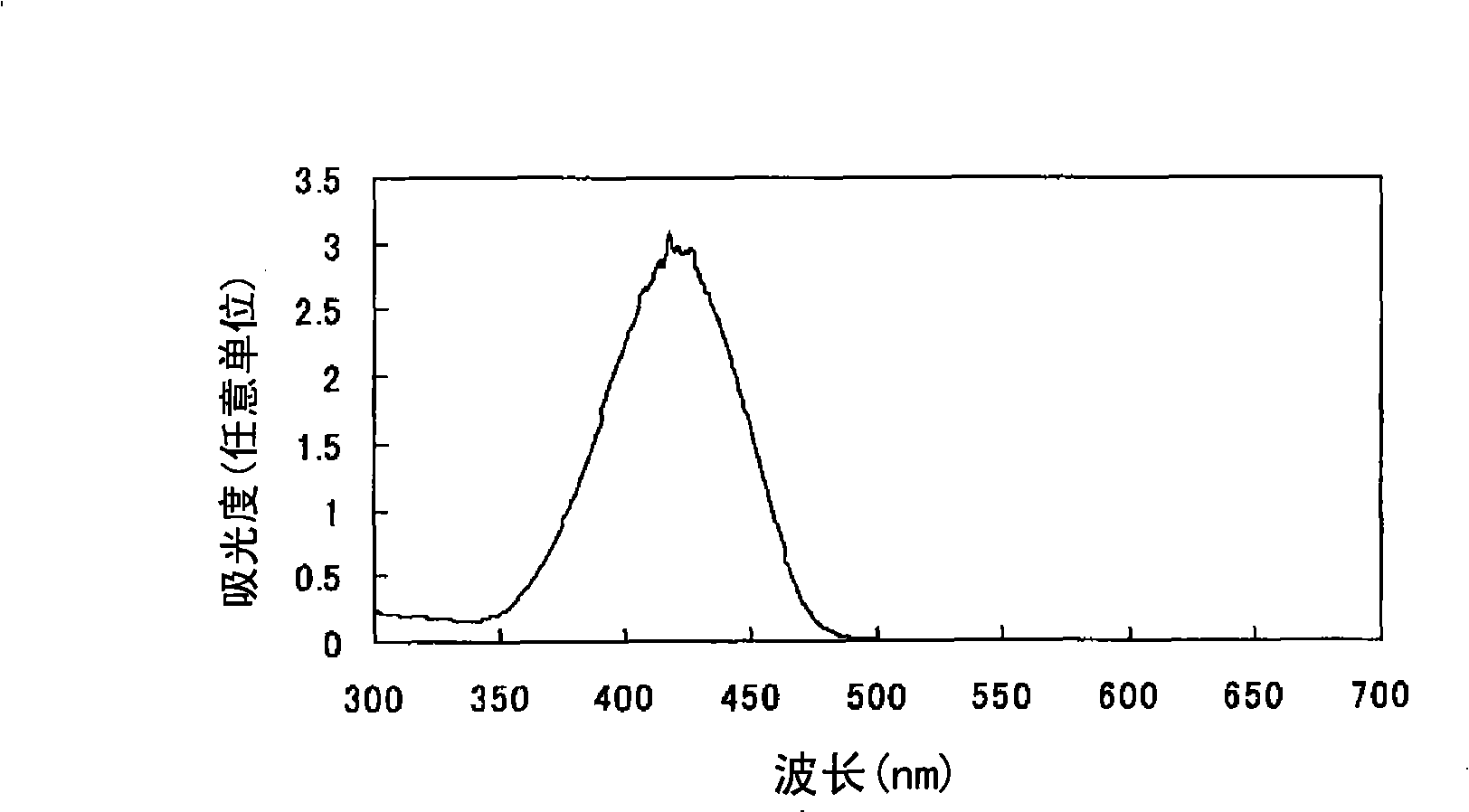
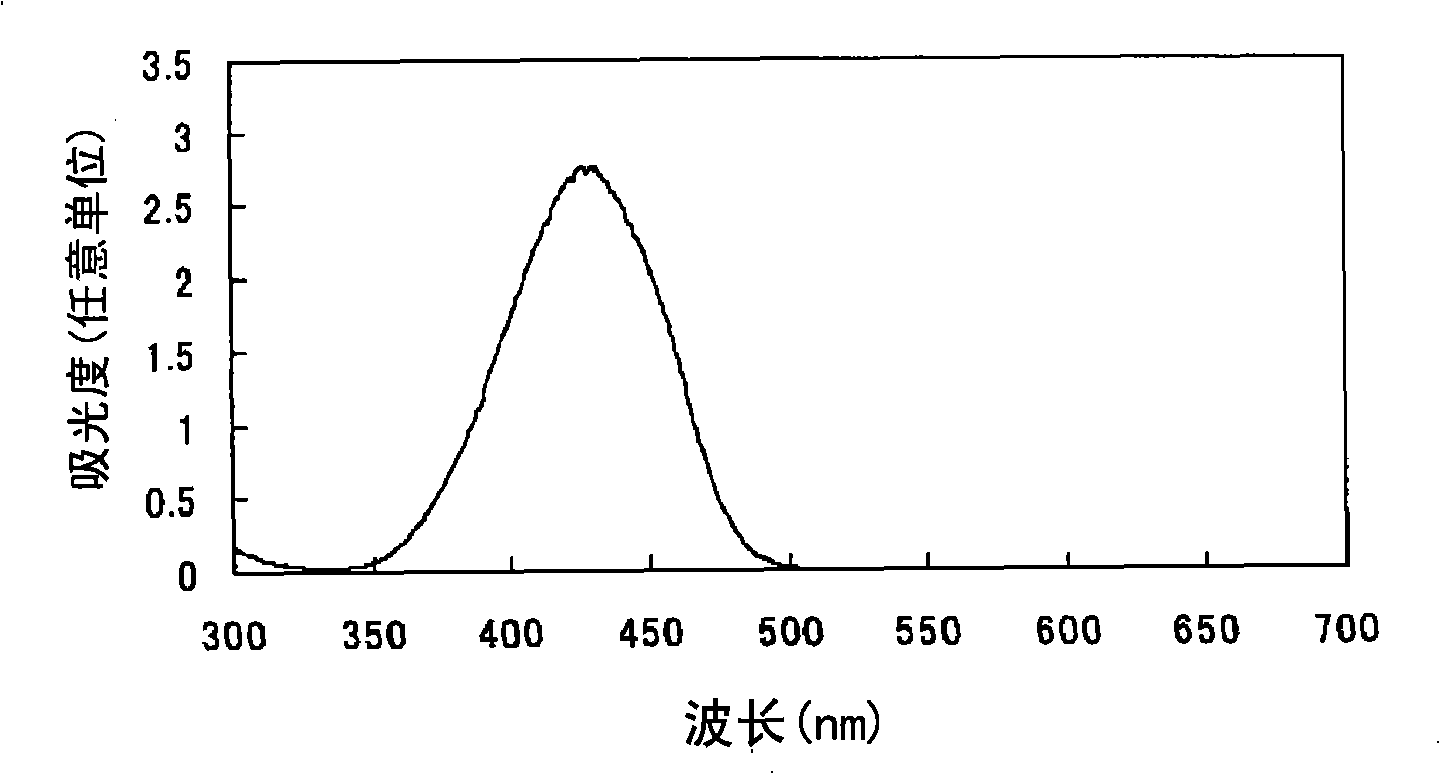
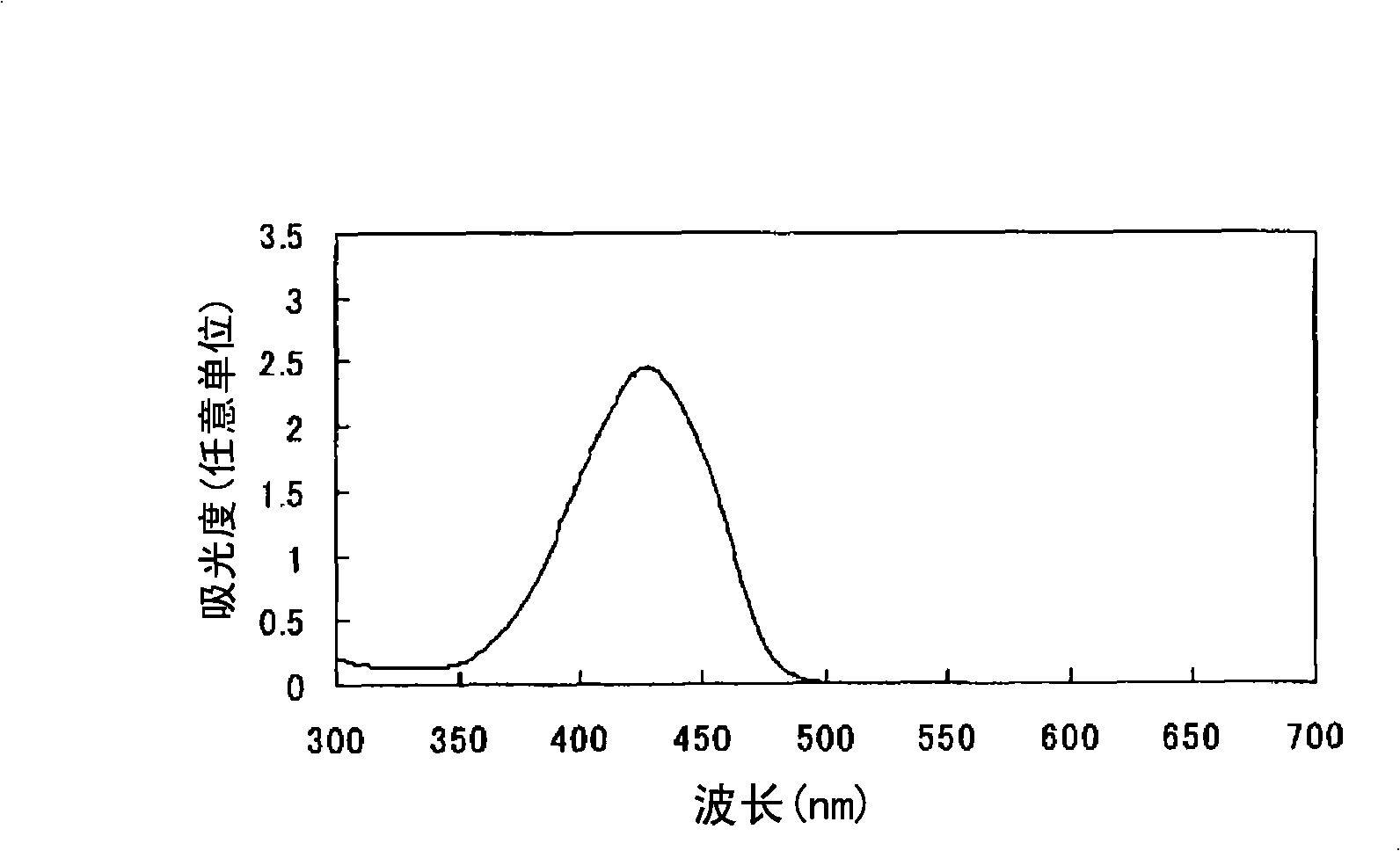
[0080] Find the oil solubility (solubility in propylene glycol monomethyl ether) of the azo compound (I-2) with the sulfo group and the azo compound (I-17) with the N-substituted sulfamoyl group according to the following method: 1 g of azo compound and 9 g of propylene glycol monomethyl ether were charged into a shaped bottle, and after stirring for a whole day and night, the remaining solid components were removed by filtration (if the azo compound was completely dissolved, the concentration of the filtrate was 10% by mass). The absorption spectrum of the filtrate obtained above was measured in the same manner as in Example 1 except that 3.5 g of the filtrate was used, and the absorbance (Int(a)) at λmax of each azo compound was determined. Moreover, the absorbance (Int(r)) at λmax of each azo compound was calculated|required similarly to Example 1 using 0.35 g of each azo compound. Then, the solubility of each azo compound was calculated from the following formula: solubili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com