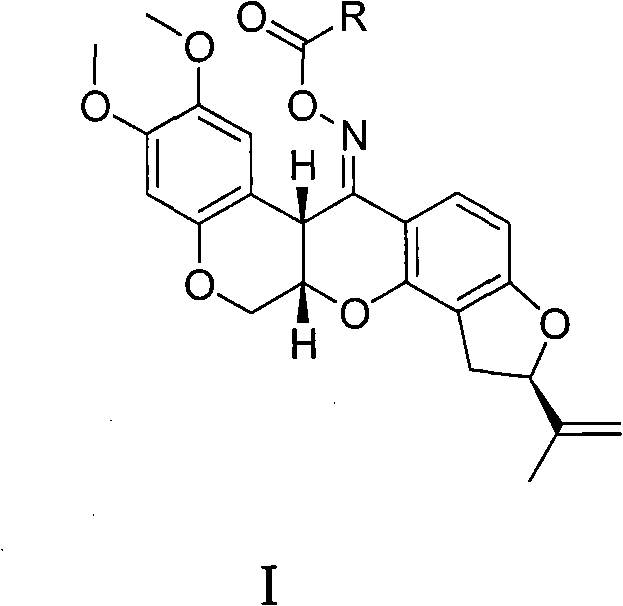
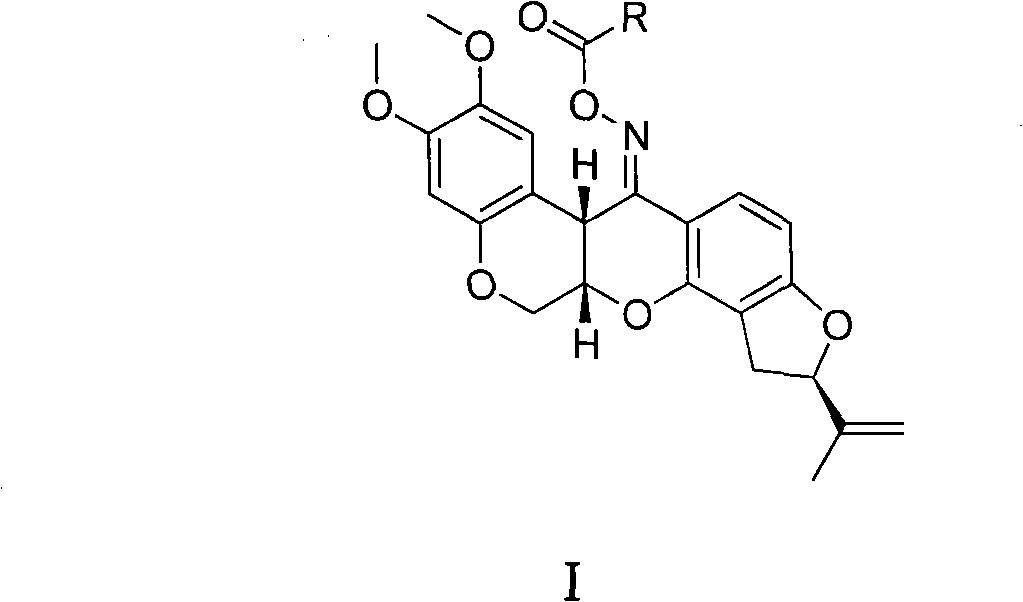
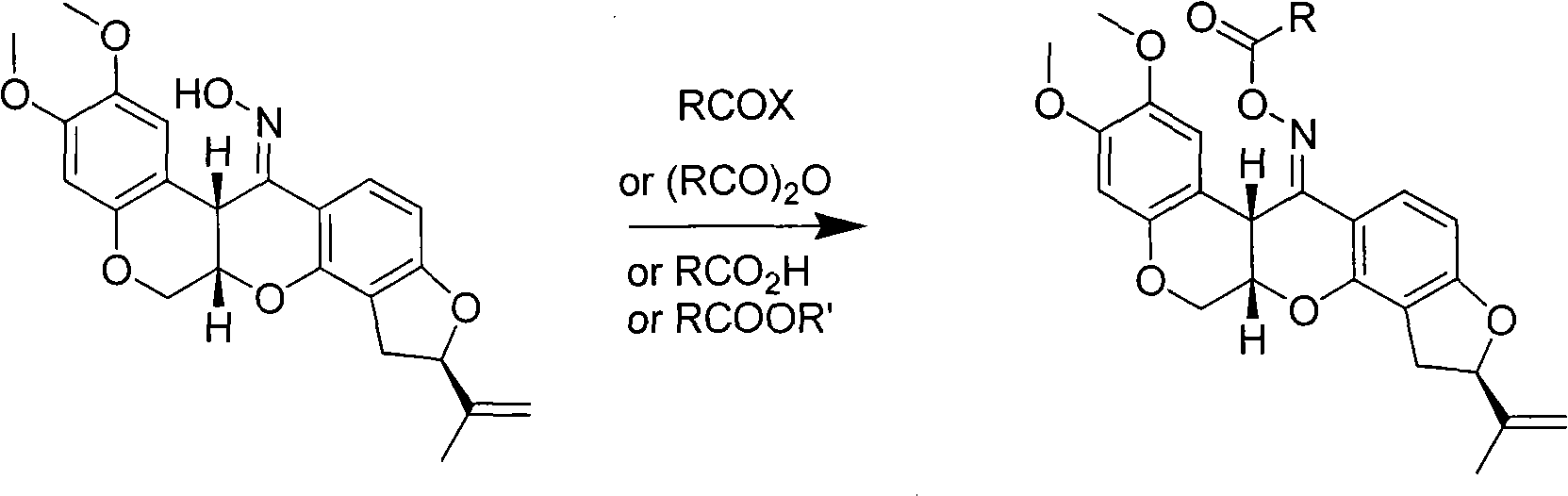Carboxylic acid rotenonoxime ester, method for preparing same and applications
A technology of rotenone oxime carboxylate and acid-binding agent, which is applied in the field of rotenone oxime carboxylate and its preparation, can solve the problems of difficult standardization, oxidative decomposition failure, and unstable preparation concentration, and achieve short reaction time and improved oxidation degradation , Synthetic operation is convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthesis of embodiment 1 rotenone oxime propionate (1)
[0019]
[0020] 20mL of acetone, 1.00g of rotenone oxime, 0.70g of potassium carbonate, 0.05g of phase transfer catalyst tetramethylammonium bromide, stirred, 0.93g of propionic anhydride was added dropwise, stirred under ice water, followed by TLC, and the reaction was completed in 2 hours. The reaction solution was filtered, the filter cake was washed with acetone, the organic phases were combined, evaporated to dryness under reduced pressure to obtain a solid, washed with water, and dried to obtain 1.10 g of a white solid, with a yield of 96.5%. m.p.206~210℃; [α] D 25 +231.3 (AcOEt). 1 H NMR (CDCl 3 ), δ: 1.25 (t, J=7.6Hz, 1H, CH 3 ), 1.76 (s, 3H, 8'-CH 3 ), 2.53 (2×q, J=7.6Hz, 1H, COCH 2 ), 2.92(dd, J=8.0Hz, J=16Hz, 1H, 4'-H), 3.28(dd, J=10.0Hz, J=16Hz, 1H, 4'-H), 3.69(s, 3H, OCH 3 ), 3.81 (s, 3H, OCH 3 ), 4.28(d, J=12Hz, 1H, 6-H), 4.58(t, J=2.8Hz, 1H, 12a-H), 4.62(dd, J=12Hz, J=2.4Hz, 1H, 6- ...
Embodiment 2
[0021] The synthesis of embodiment 2 rotenone oxime propionate (1)
[0022] 20mL of N,N-dimethylformamide, 1.00g of rotenone oxime, 0.4g of pyridine, stirred for 30min, added dropwise 0.27g of propionic acid, stirred at 150°C, followed by TLC, and the reaction was completed in 2h. The reaction solution was filtered, the filter cake was washed with acetone, the organic phases were combined, evaporated to dryness under reduced pressure to obtain a solid, washed with water, and dried to obtain a white solid, m.p.206-209°C; [α] D 25 +231.3 (AcOEt), the spectrogram data is consistent with Example 1.
Embodiment 3
[0023] The synthesis of embodiment 3 rotenone oxime propionate (1)
[0024] 20mL chloroform, 1.00g rotenone oxime, 0.50g sodium carbonate, 0.05g phase transfer catalyst tetramethylammonium chloride, 0.93g propionic anhydride was added dropwise, stirred at 60°C, followed by TLC, and the reaction was completed in 2.5h. The reaction solution was filtered, the filter cake was washed with acetone, the organic phases were combined, evaporated to dryness under reduced pressure to obtain a solid, washed with water, and dried to obtain 8.8 g of a white solid, with a yield of 77.2%. m.p.206~210℃; [α] D 25 +231.3 (AcOEt), the spectrogram data is consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com