Temperature sensing in situ gel rubber formulations capable of being injected, preparation method and uses thereof
An in-situ gel and preparation technology, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of paclitaxel injection application limitation, large toxic side effects, vasodilation, etc., To achieve the effect of improving medication compliance, high practicability, and maintaining long-term effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
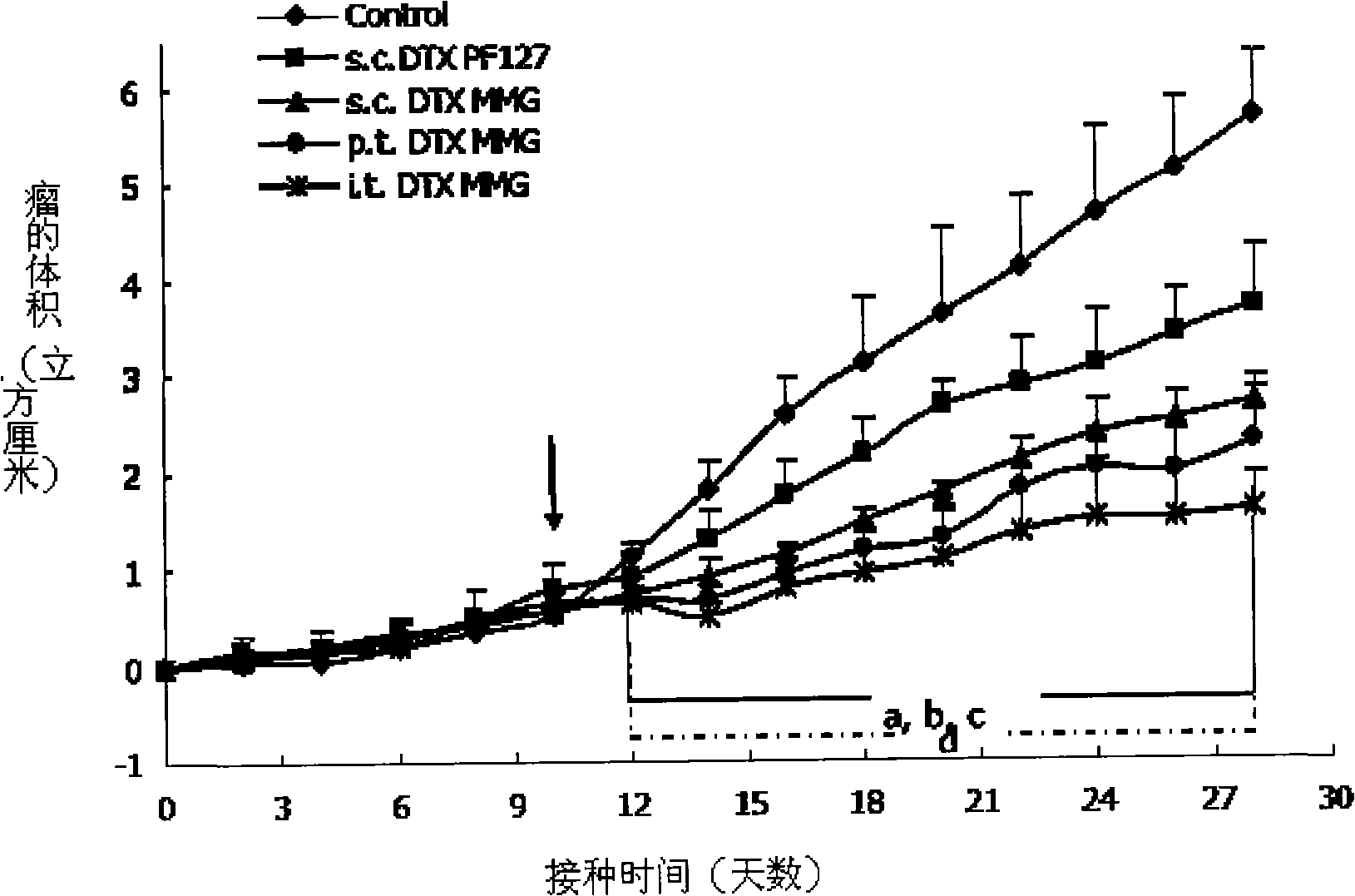
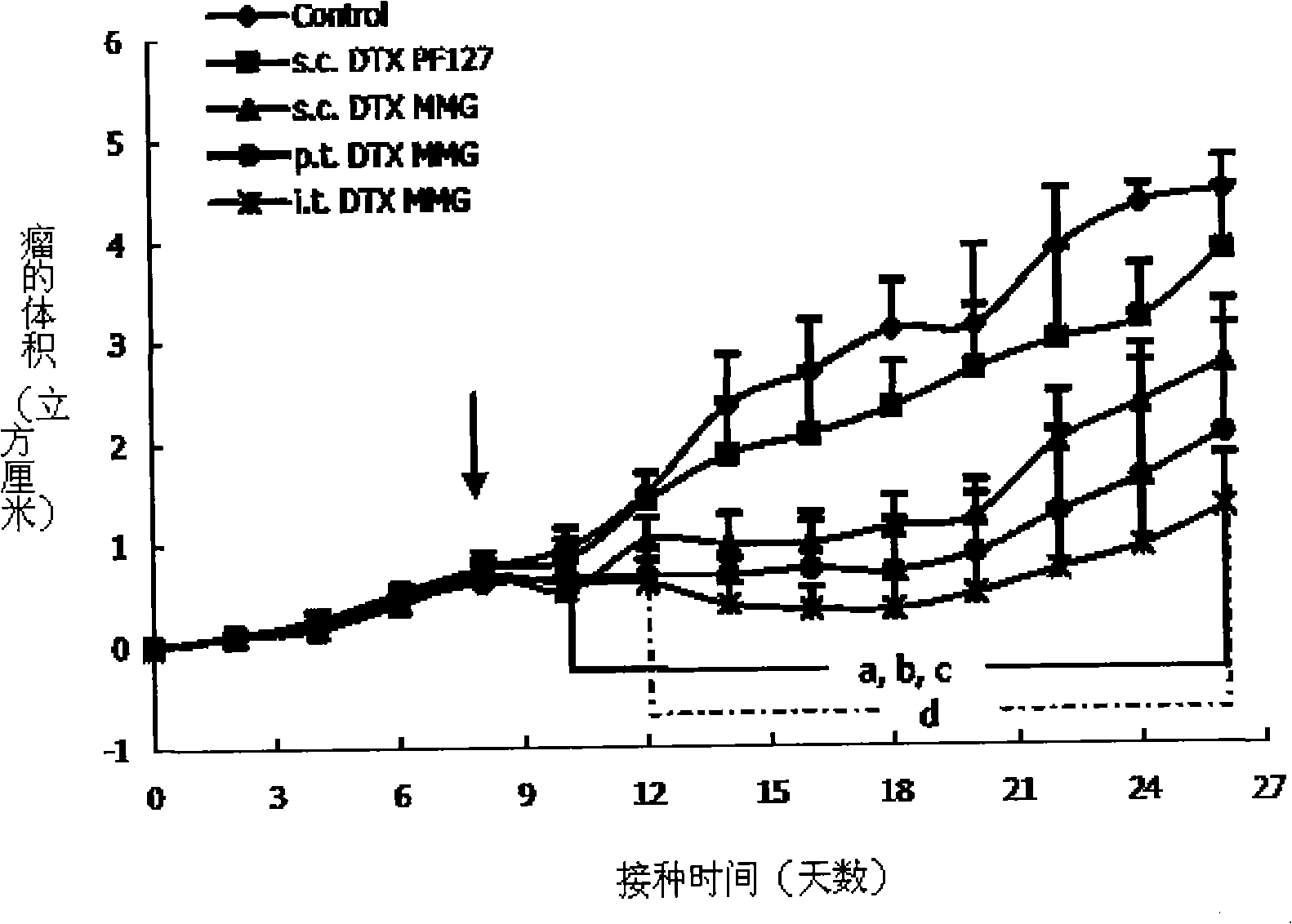
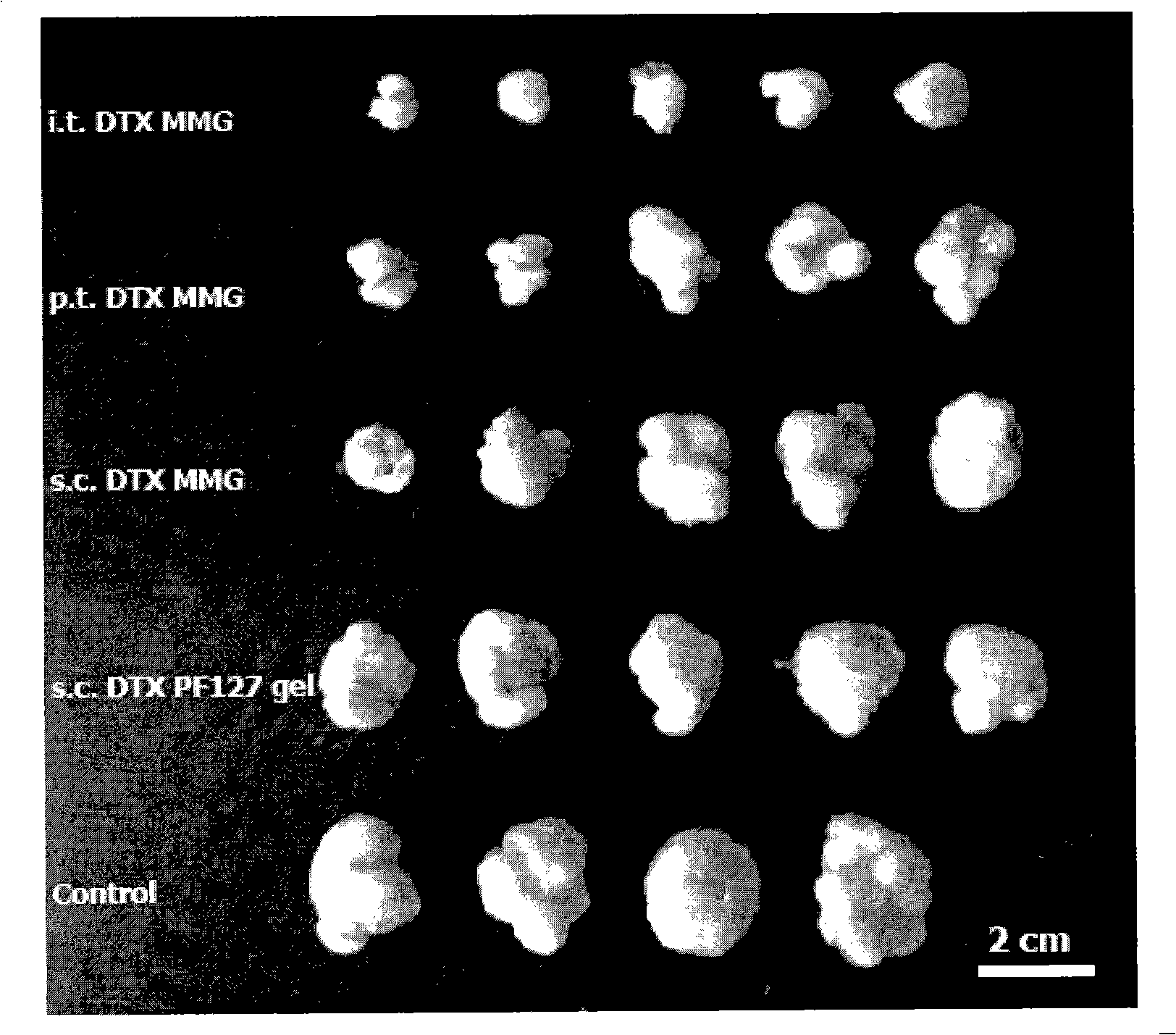
Image
Examples
Embodiment 1
[0033] Take an appropriate amount of Pluronic F127, so that the final concentration is controlled at 20 wt %; add an appropriate amount of Tween 80, so that the molar ratio of it to F127 is 6:1. The final concentration of docetaxel (DTX) added was 2 mg / ml, the carrier material and the drug were placed in a vial, an appropriate proportion of distilled water was added, and magnetically stirred overnight (12 h) in an ice-bathed beaker at 4°C. A translucent drug-loaded sol system is formed.
Embodiment 2
[0035] Take an appropriate amount of Pluronic F127 to make the final concentration 20% by weight; add an appropriate amount of Tween 80 to make the final concentration 15%. The final concentration of paclitaxel (PTX) added was 2 mg / ml; the polymer materials and drugs were placed in vials, an appropriate proportion of distilled water was added, and magnetically stirred overnight (12 h) in an ice-bathed beaker at 4°C. A translucent drug-loaded sol system is formed.
Embodiment 3
[0037] Take an appropriate amount of Pluronic F127 to make the final concentration 20% by weight; add an appropriate amount of Tween 80 to make the final concentration 10%. The final concentration of 9-nitrocamptothecin (9-NC) added was 4 mg / ml; polymer materials and drugs were placed in vials, an appropriate proportion of distilled water was added, and magnetically stirred in an ice-bathed beaker at 4°C overnight (12h ). A translucent drug-loaded sol system is formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com