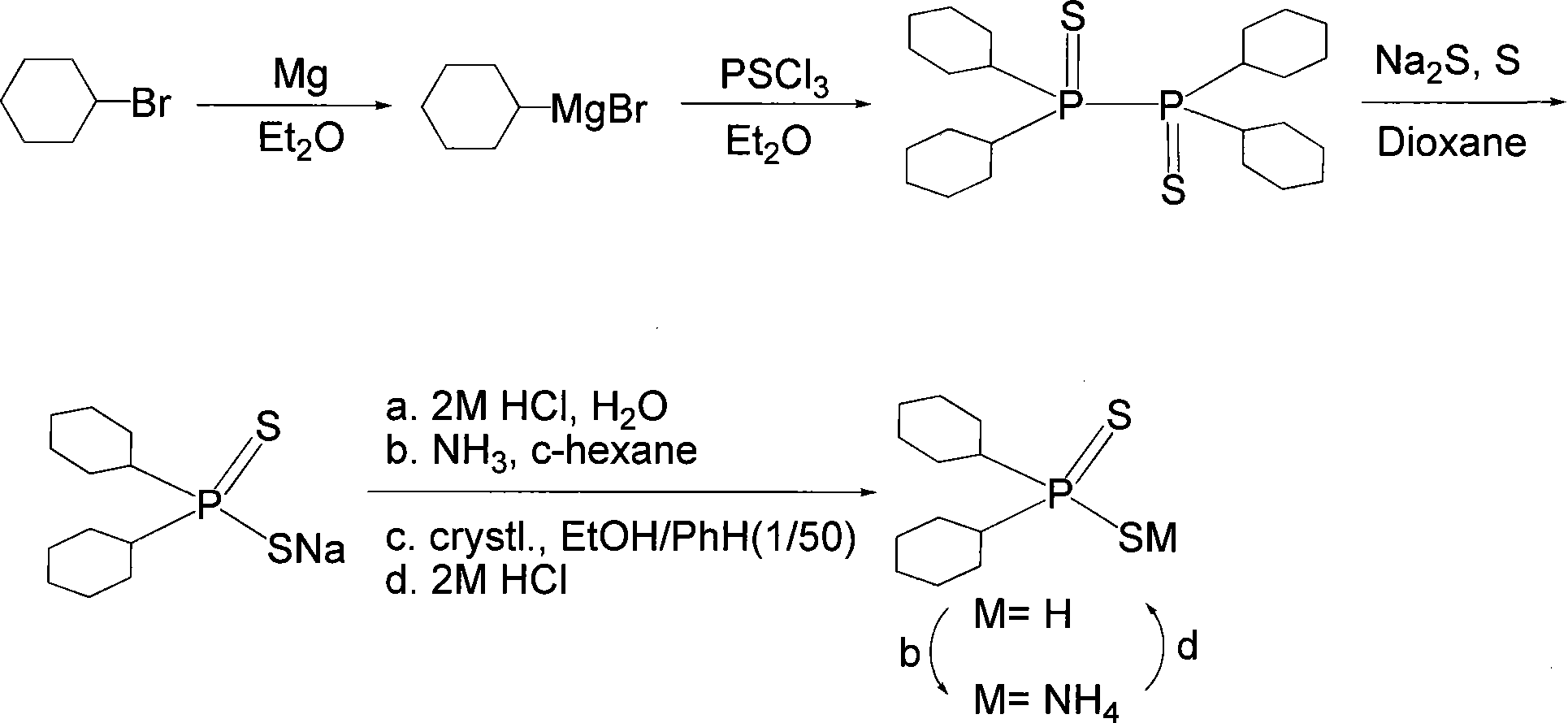
Synthesis of sicycle-ring hexyl di-thiophosphinic acid
A technology of dicyclohexyl dithiophosphinic acid and a synthesis method, which is applied in chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, organic chemistry, etc., can solve the problems of volatility, strong irritation and toxicity It can achieve the effects of low toxicity, high yield, and cheap and easy-to-obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0018] The preparation of embodiment 1 dicyclohexyl dithiophosphinic acid
[0019] Follow the steps below:
[0020] (1) Preparation of Grignard reagent: 12.2 (0.5 mol) g of magnesium strips, 20 ml of anhydrous ether and 3 iodines are placed in a 500 ml three-necked flask equipped with a sealed stirrer and a reflux condenser with a drying tube, and Mix 61ml (0.5mol) of bromocyclohexane and 130ml of anhydrous ether in the pressure dropping funnel; first dropwise add 20ml of bromocyclohexane ether solution, let stand to initiate the reaction; then start stirring and add the remaining bromocyclohexane dropwise Cyclohexane ether solution, keep the reaction mixture at a gentle reflux; after the dropwise addition, heat the reaction mixture to reflux until the magnesium strips basically disappear, then cool in an ice bath to obtain a gray clear liquid, which is the Grignard reagent.
[0021] (2) Preparation of biphosphine intermediate: Cool the Grignard reagent obtained in step (1) t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com