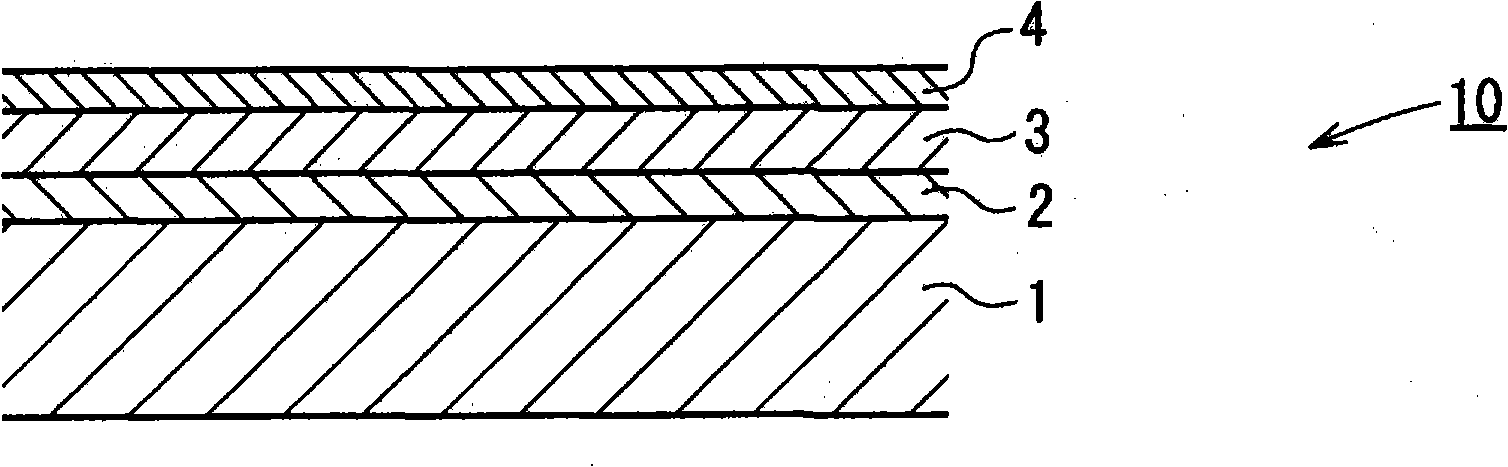
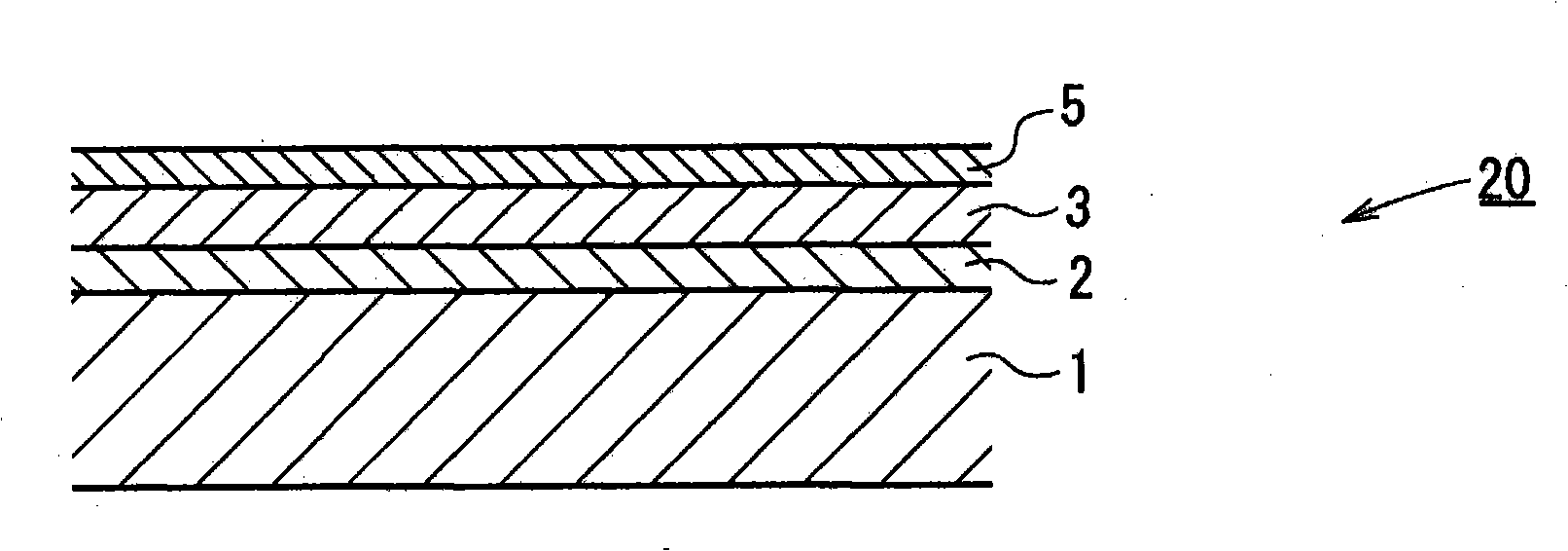
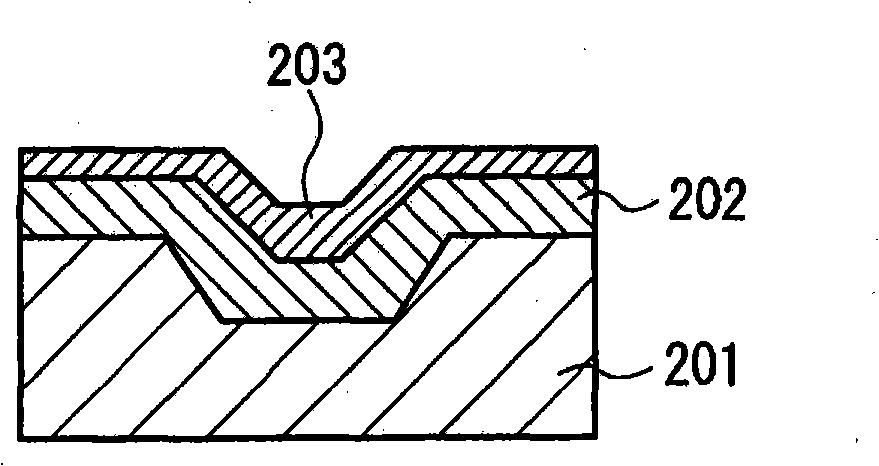
Hydrazide chelate complex compound, and optical recording medium using the compound, and its recording method
An optical recording medium, a technology of coordination compounds, applied in the directions of optical recording carriers, chemical instruments and methods, temperature recording methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0285]
[0286]
[0287] 2-(1,3,3-Trimethylindolin-2-ylidene)acetaldehyde (1.0 g) and benzohydrazide (0.70 g) were heated and stirred in ethanol (20 ml) for 1 hour. The reaction mixture was cooled to room temperature, water (20ml) was added and the resulting solid was filtered. Wash with water (10 ml) and ethyl acetate (10 ml), and dry to obtain the light yellow target compound (L-1) (1.3 g, yield 82%).
[0288] pass 1 The obtained compound was identified by H NMR.
[0289] NMR (CDCl 3 )δ: 8.57(d, J=10.0Hz, 1H), 7.85(d, J=8.0Hz, 2H), 7.5-7.4(m, 3H), 7.25-7.1(m, 2H), 6.90(t, J =8.0Hz, 1H), 6.68(d, J=8.0Hz, 1H), 5.60(d, J=10.0Hz, 1H), 3.22(s, 3H), 1.57(s, 6H)
[0290]
[0291] Compound (L-1) (0.50 g) was dissolved in hot methanol (20 ml). Triethylamine (0.16 g) and cobalt acetate tetrahydrate (0.20 g) were successively added to this solution, followed by stirring at 50° C. for 1 hour. The reaction mixture was filtered, the filtrate was added dropwis...
Embodiment 2
[0299]
[0300] Compound (L-1) (0.25 g) was dissolved in hot methanol (20 ml). Triethylamine (90 mg) and copper acetate tetrahydrate (0.10 g) were successively added to this solution, followed by stirring at 50° C. for 1 hour. Water (20ml) was added to the reaction mixture, the resulting solid was filtered, washed with water (10ml) and methanol (10ml) to obtain the yellow solid (0.20g, yield 82%) of the following target compound (A-2) .
[0301] λmax (CH 3 CN): 415nm
[0302] OD of λmax: 135
[0303]
[0304] The obtained compound (A-2) was tested for its solubility in a coating solvent by the method shown in Example 1. As a result, it was found that the compound (A-2) was completely dissolved at a concentration of 0.7% by weight. insoluble components.
Embodiment 3
[0306]
[0307]
[0308] In methanol ( 30ml) was heated and stirred for 1 hour. The solvent was distilled off from the reaction mixture under reduced pressure. After extraction with ethyl acetate and washing with brine, the organic layer was dried over magnesium sulfate, and the solvent was distilled off under reduced pressure. This was recrystallized from chloroform and hexane to obtain a light yellow target compound (L-2) (0.62 g, yield 38%).
[0309] The obtained compounds were identified by DEI-MS.
[0310] DEI-MS (measured value): 329 (M + ), calculated value: 329
[0311]
[0312] Compound (L-2) (0.25 g) was dissolved in hot methanol (20 ml). Triethylamine (0.30 g) and cobalt acetate tetrahydrate (0.37 g) were successively added to this solution, followed by stirring at a temperature of 50° C. for 1 hour. The reaction mixture was filtered, the filtrate was added dropwise in water (20ml), the solid generated by filtration was washed with water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com