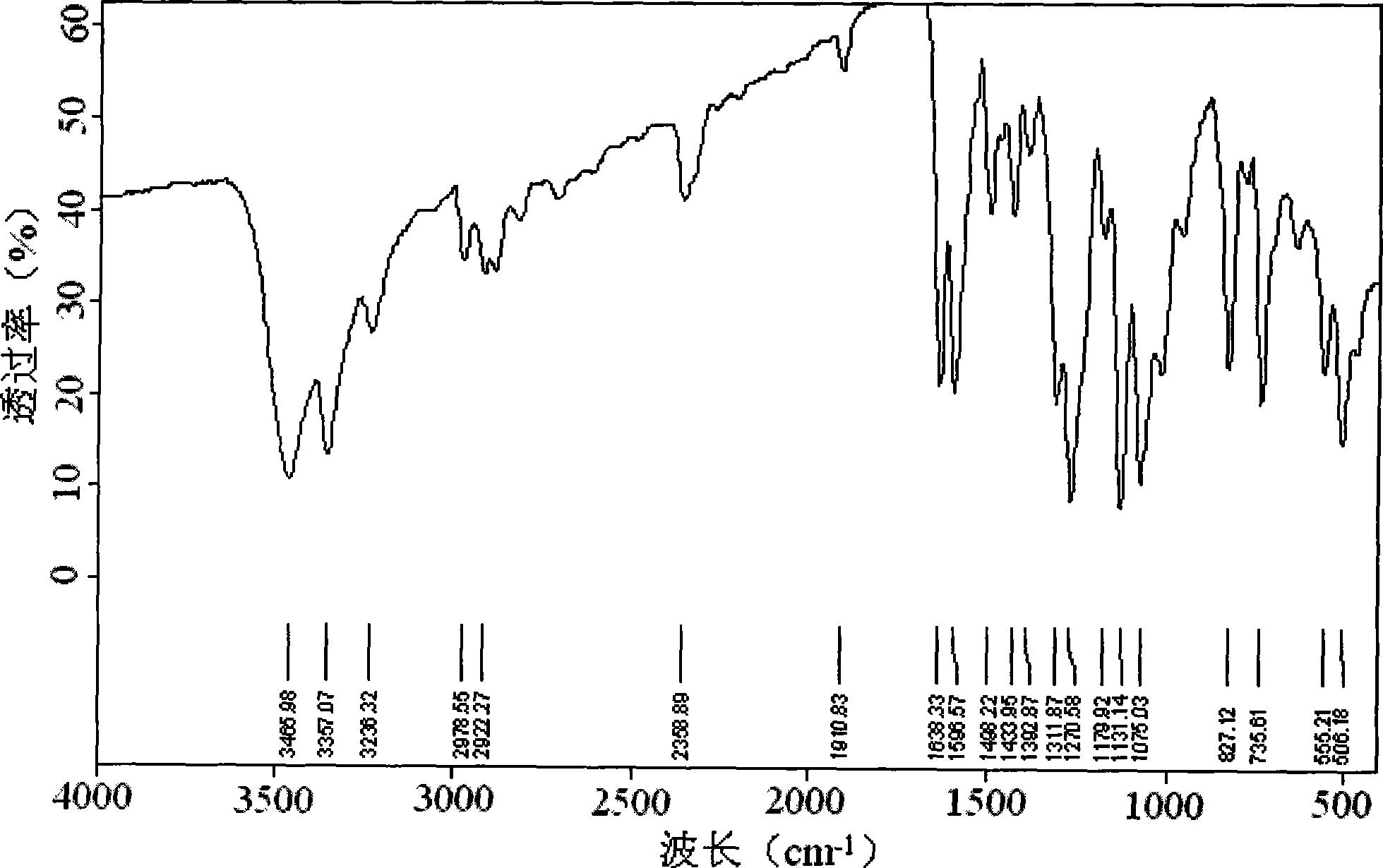
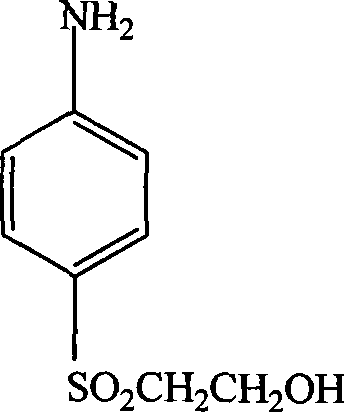
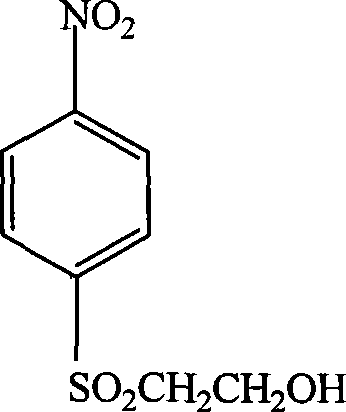P-aminophenyl-beta-hydroxyethyl sulfone preparation method
A technology of p-aminophenyl and hydroxyethyl sulfone, which is applied in the field of preparation of β-hydroxyethyl sulfone, can solve the problems of difficult catalyst recovery, high production cost, and low product yield, and achieve easy recovery and short reaction time , the effect of small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0013] Specific embodiment one: the preparation method of p-aminophenyl-β-hydroxyethyl sulfone in this embodiment is completed by the following reaction: p-nitrophenyl-β-hydroxyethyl sulfide, nickel aluminum alloy catalyst Mix with solvent, pass in nitrogen to remove the air, then pass in hydrogen to control the pressure of hydrogen to 0.5~6MPa, while stirring at a speed of 500~1000r / min, and raise the temperature to 40~100°C, react for 4~12h, then filter and distill and drying to obtain p-aminophenyl-beta-hydroxyethyl sulfone; wherein the consumption of nickel-aluminum alloy catalyst is 0.5%~5% of p-nitrophenyl-beta-hydroxyethyl sulfide quality, and the solvent consumption is p-nitrophenyl 1 to 5 times the mass of phenyl-β-hydroxyethyl sulfide; the solvent is one of water, methanol, ethanol, propanol, butanol, dimethylformamide or a mixture of several of them.
[0014] In this embodiment, when the solvent is a mixture, various mixtures are mixed in any ratio. In this embodim...
specific Embodiment approach 2
[0019] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the nickel-aluminum alloy catalyst is made by the following method: 1. The nickel-aluminum alloy powder containing 40% to 70% nickel, organic Adhesives, inorganic adhesives, extrusion aids and peptizers are mixed to obtain jelly; 2. The jelly is directly kneaded and shaped, dried under an infrared lamp, and then dried at 120°C for 12 hours, and then Roast at 400-450°C for 1-3 hours, heat up to 500-600°C for 2-4 hours, and heat up at 800-900°C for 2-5 hours; 3. Crush the product in step 2 and sieve out a particle size of 40 ~60 mesh particles, the sieved particles are leached in the activator at 50~90°C for 6~36h, the stirring speed is 50~80r / min during the leaching process, and the water is continuously replenished during the leaching process to maintain a constant solution volume, and then Washing with deoxidized and deionized water until the pH value of the leached water is 7 to 8 to obtain ...
specific Embodiment approach 3
[0021] Embodiment 3: This embodiment is different from Embodiment 2 in that the molecular weight of the organic binder is 30,000-300,000. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com