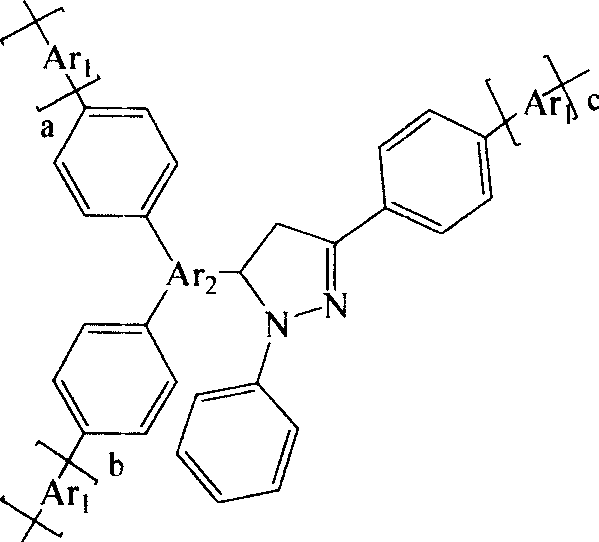
Blue hyper-branched polymer electroluminescent materials using pyrazoline unit as nuclear and preparation method thereof
A technology of hyperbranched macromolecules and electroluminescent materials, applied in luminescent materials, electroluminescent light sources, chemical instruments and methods, etc., can solve the problems of reducing device efficiency and luminescent color purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
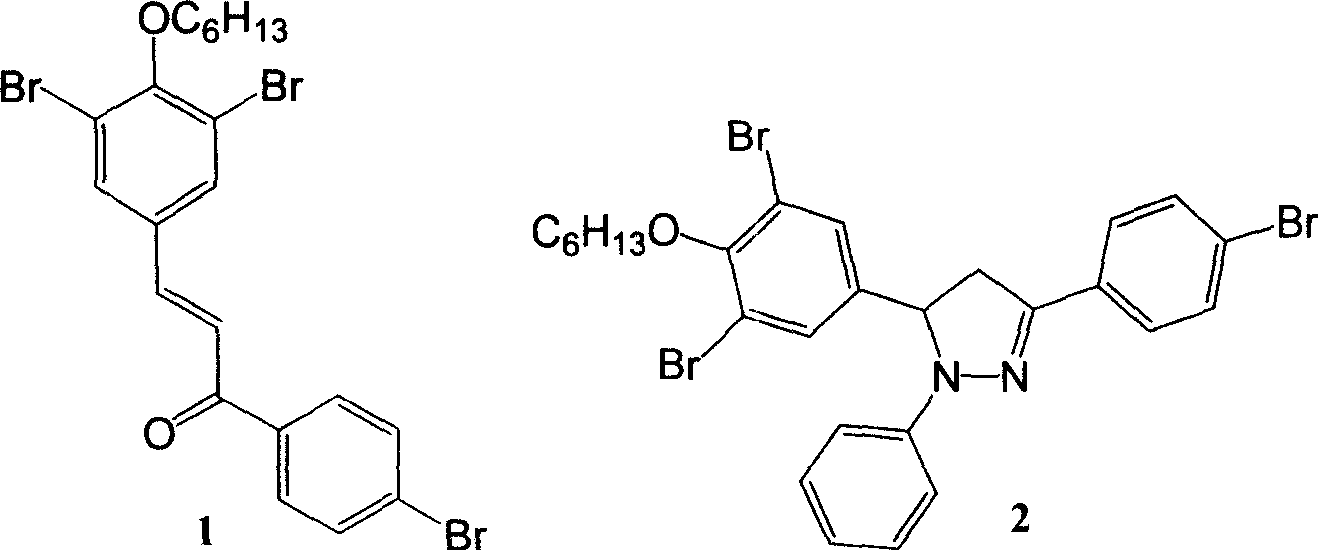
[0034] Embodiment one is: the synthesis of structural formula 1
[0035] In a 50 ml three-necked flask, add 0.78 ml of 6.85 mol / liter sodium hydroxide solution, 0.93 g (4.67 mmol) of p-bromoacetophenone, 1.70 g (4.67 mmol) of 3,5-dibromo-4- Hexyloxybenzaldehyde and 17 ml of ethanol form a mixed solution. After stirring the reaction mixture at room temperature for 5 hours, it was filtered, and the precipitate was washed with pure water and methanol, respectively. The primary product was recrystallized in ethanol to obtain a pure product with a yield of 83%. 1 HNMR (CDCl 3 , 300MH z , δ / ppm): 7.90-7.87 (d, 2H, Ar-H), 7.77 (s, 1H, CO-C=CH), 7.67-7.61 (m, 3H, Ar-H), 7.40 (s, 1H , CO-CH=C), 4.07-4.03(t, 2H, O-CH 2 ), 1.94-0.90 (m, 11H, CH 2 and CH 3 ).
Embodiment 2
[0036] Embodiment two is: the synthesis of structural formula 2
[0037] In a 50 mL round bottom flask, 1.71 g (3.13 mmol) of intermediate 1, 0.34 g (3.13 mmol) of phenylhydrazine and 12 mL of ethylene glycol monoethyl ether were added. The reaction mixture was heated to reflux under the protection of argon for 3 hours and then cooled to room temperature. Filter, wash the precipitate with water, and dry. The primary product was recrystallized in ethanol to obtain a pure product with a yield of 85%. 1 H NMR (CDCl 3 , 300MH z , δ / ppm): 7.58-7.49 (m, 4H, Ar-H), 7.45 (s, 1H, Ar-H), 7.25-7.19 (t, 2H, Ar-H), 7.04-7.02 (d, 2H , Ar-H), 6.87-6.83(t, 1H, Ar-H), 5.18-5.12(m, 1H, pyrazoline ring CH), 4.00-3.96(t, 2H, O-CH 2 ), 3.85-3.75 (m, 1H, pyrazoline ring CH 2 ), 3.11-3.03 (m, 1H, pyrazoline ring CH 2 ), 1.90-0.88 (m, 11H, CH 2 and CH 3 ).
Embodiment 3
[0038] Embodiment three is: the synthesis of structural formula 3
[0039] In a 50 ml three-necked flask, add 0.78 ml of 6.85 mol / liter sodium hydroxide solution, 0.93 g (4.67 mmol) of p-bromoacetophenone, 2.01 g (4.67 mmol) of 4-bis-p-bromoanilino benzaldehyde and 17 ml of ethanol to form a mixed solution. After stirring the reaction mixture at room temperature for 5 hours, it was filtered, and the precipitate was washed with pure water and methanol, respectively. The primary product was recrystallized in ethanol to obtain a pure product with a yield of 80%. 1 H NMR (CDCl 3 , 300MHz, δ / ppm): 7.86-7.85(d, 2H), 7.78-7.73(d, 1H, CO-C=CH), 7.65-7.62(d, 2H, Ar-H), 7.52-7.49(d , 2H, Ar-H), 7.43-7.37(m, 4H, Ar-H), 7.32(s, 1H, CO-CH=C), 7.04-6.97(m, 6H, Ar-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com