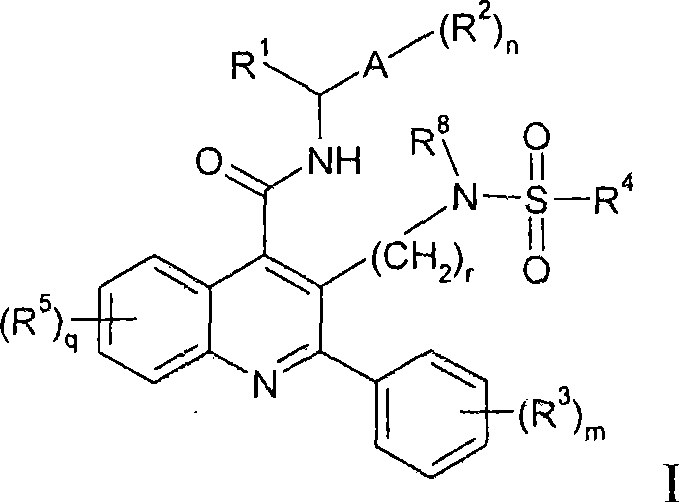
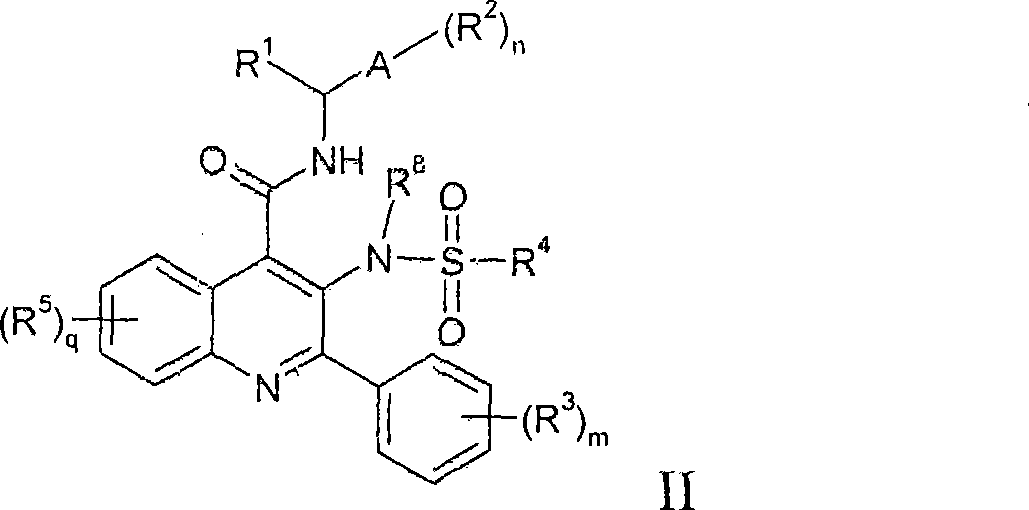
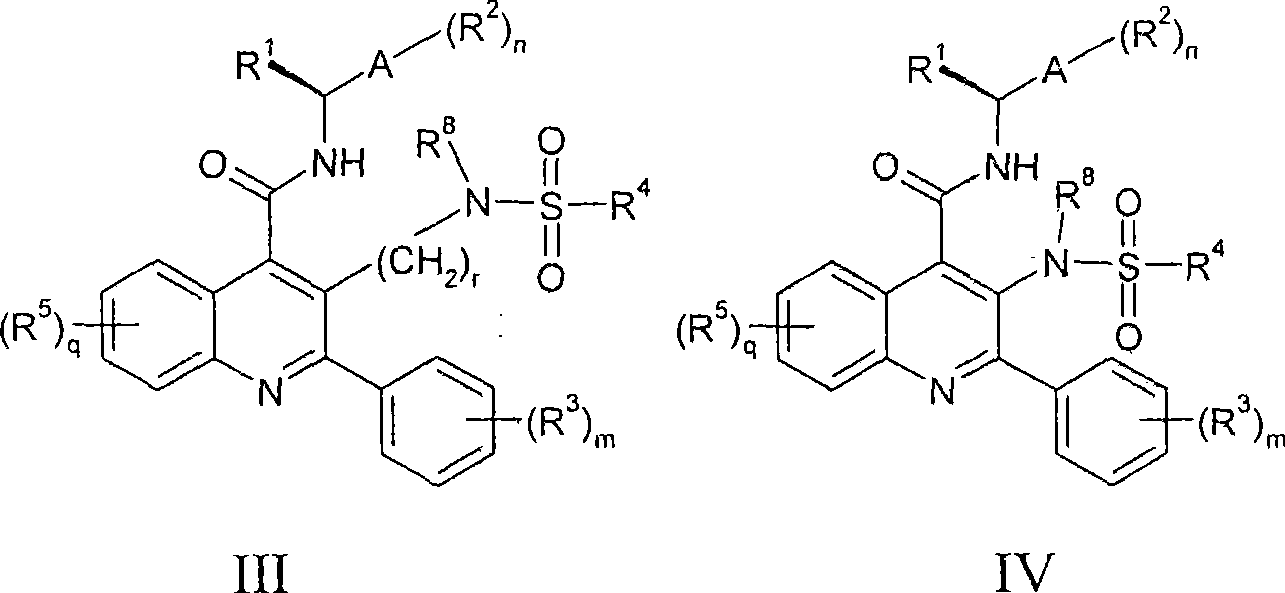
Alkylsulphonamide quinolines
An alkyl and amide technology, applied in the field of quinoline derivatives, can solve problems such as limited evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1. 3-[(methylsulfonyl)amino]-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-formyl Amines (1) (embodiment 1 relates to scheme 1)
[0180]
[0181] EDCl (289 mg, 1.5 mmol) was added to 3-[(methylsulfonyl)amino]-2-phenylquinoline-4-carboxylic acid (1c) (342 mg, 1.0 mmol), HOBT under nitrogen atmosphere at room temperature Hydrate (231 mg, 1.5 mmol), 4-methylmorpholine (276 μL, 1.5 mmol) in tetrahydrofuran (50 ml). Then (S)-1-phenylpropylamine (135 mg, 1.0 mmol) was added and the reaction mixture was stirred at room temperature for 12 hours. All solvent was removed in vacuo, and the residue was partitioned between ethyl acetate and 10% aqueous sodium bicarbonate, dried over sodium sulfate, and concentrated in vacuo. The residue was purified by chromatography (eluting with 15-25% ethyl acetate / hexanes) to afford the title compound (70 mg, 15%) as a solid. 1 HNMR (300MHz, CDCl 3 )δ 0.94(t, 3H), 1.97(m, 2H), 3.44(s, 3H), 5.17(q, 1H), 5.47(m, 2H), 7.32(d, 2H),...
Embodiment 2
[0187] Example 2: 3-(methylsulfonylamino-methyl)-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propane base)-amide (Example 2 and 3 relate to scheme 2)
[0188]
[0189] Under a nitrogen atmosphere, triethylamine (140 μL, 1.0 mmol) was added to 3-(aminomethyl)-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-methanol Amide (2e) (197mg, 0.5mmol) in DCM (30ml). While cooling in an ice-water bath, methanesulfonyl chloride (39 μL, 0.51 mmol) was added dropwise, and the reaction mixture was stirred at room temperature for an additional 2 hours. The mixture was washed with brine (10 mL), the organic phase was separated, dried over sodium sulfate and concentrated in vacuo. The resulting residue was purified by chromatography (eluting with 15-25% ethyl acetate / hexanes) to afford the title compound (79 mg, 42%) as a solid. 1 HNMR (300MHz, CDCl 3 )δ 0.94(t, 3H), 1.95(m, 2H), 2.99(s, 3H), 4.88(s, 2H), 5.25(q, 1H), 6.66(b, 2H), 7.30(d, 2H) , 7.34(d, 2H), 7.39(m, 1H), 7.78(m, ...
Embodiment 3
[0190] Example 3. 3-{[(ethylsulfonyl)amino]methyl}-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline -4-formamide
[0191]
[0192] Using a procedure similar to that described in Example 2, except using the component ethanesulfonyl chloride (48.6 μL, 0.51 mmol), the title compound was obtained as a white solid (90 mg, 38%). 1 HNMR (300MHz, CDCl 3 )δ 0.92(t, 3H), 1.32(t, 3H), 1.95(m, 2H), 3.04(q, 2H), 4.87(s, 2H), 5.17(q, 1H), 5.48(b, 2H) , 7.25(d, 2H), 7.34(d, 2H), 7.39(m, 1H), 7.78(m, 2H), 7.84(m, 2H), 8.08(m, 1H), 8.30(m, 2H), 8.17 (m, 2H). MSAPCI, m / z=488 (M+1). LCMS: 2.24 minutes.
[0193] The starting amine used in Examples 2 and 3, 3-(aminomethyl)-2-phenyl-N-[(1S)-1-phenylpropyl]quinoline-4-carboxamide, was prepared as follows:
[0194] 3-(Azidomethyl)-2-phenylquinoline-4-carboxylic acid methyl ester (2b)
[0195] Sodium azide (402 mg, 6.18 mmol) was added to 3-(bromomethyl)-2-phenylquinoline-4-carboxylic acid methyl ester (2a) (2000 mg, 5.618 mmol) in THF / DMF (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com