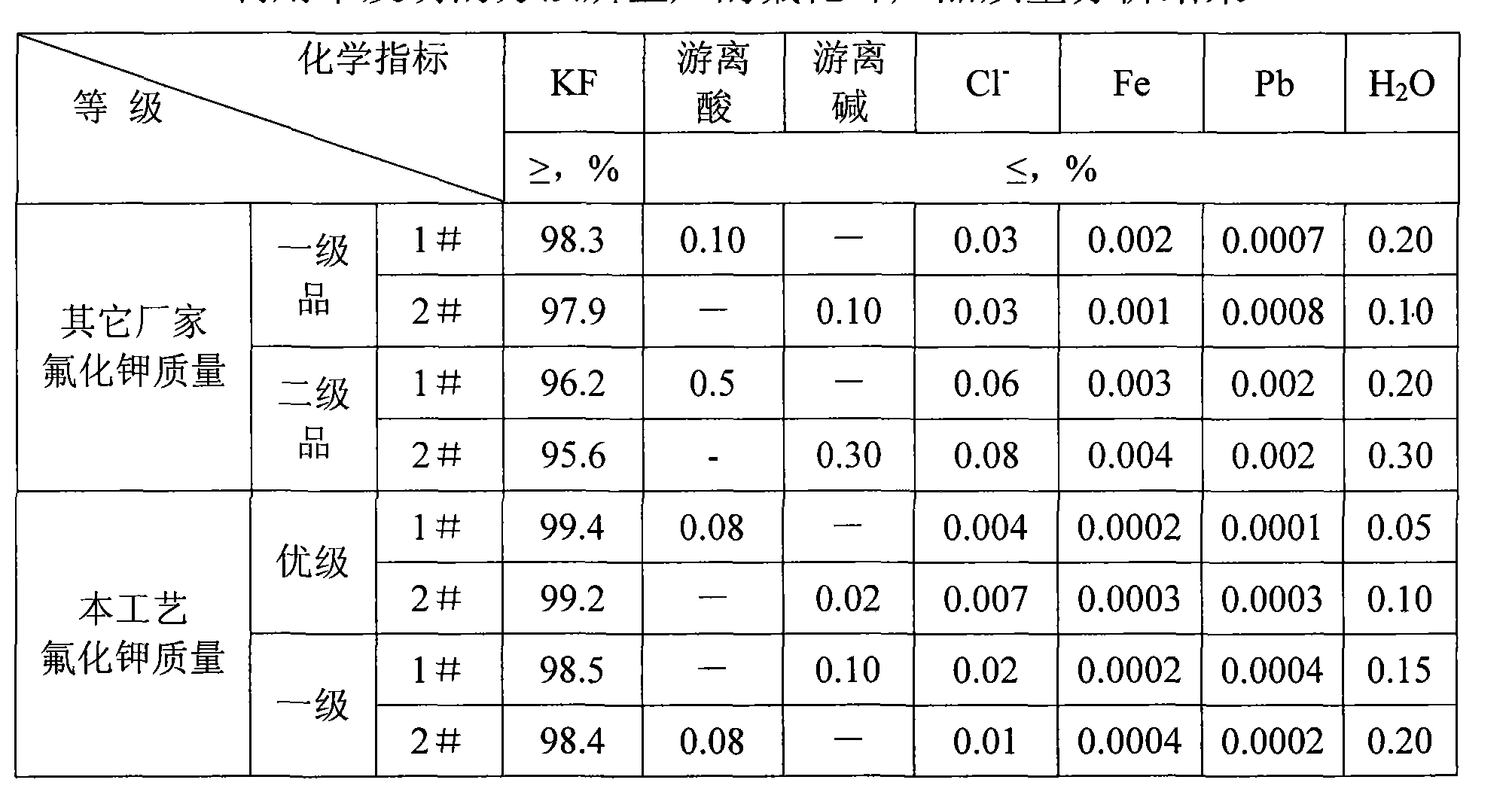
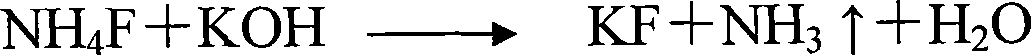Method for preparing potassium fluoride
A technology of potassium fluoride and ammonium fluoride, applied in the direction of alkali metal fluoride, etc., can solve the problems of rising hydrofluoric acid price and affecting the production cost of potassium fluoride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The method for preparing potassium fluoride in this embodiment uses ammonium fluoride and potassium hydroxide as main raw materials, and specifically comprises the following steps:
[0021] (1) Pour 20% ammonium fluoride solution into the reaction kettle, start stirring, add the required solid potassium hydroxide in an amount of 1.10 times ammonium fluoride at a uniform speed, the pH value at the end of the reaction is 8, and the reaction is put Thermal reaction, after the reaction, the temperature reaches above 110°C; the water vapor is cooled, gas-liquid separation, and the liquid enters the condensation tank; the gas is absorbed into ammonia water through the cooling cycle, enters the storage tank, and the rest of the gas is emptied;
[0022] (2) After the reaction in the reactor is complete, the liquid flows into the concentration tank, the concentrated water vapor is cooled and separated from gas and liquid, and the liquid enters the condensation tank; the gas is co...
Embodiment 2
[0026] The method for preparing potassium fluoride in this embodiment uses ammonium fluoride and potassium hydroxide as main raw materials, and specifically comprises the following steps:
[0027] (1) Pour 30% ammonium fluoride solution into the reaction kettle, start stirring, add the required solid potassium hydroxide according to the addition of 0.9 times of ammonium fluoride at a uniform speed, the pH value at the end of the reaction is 7.5, and the reaction is Exothermic reaction, after the reaction, the temperature reaches above 110°C; the water vapor is cooled, gas-liquid separation, and the liquid enters the condensation tank; the gas is absorbed into ammonia water through the cooling cycle, enters the storage tank, and the rest of the gas is evacuated;
[0028] (2) After the reaction in the reactor is complete, the liquid flows into the concentration tank, the concentrated water vapor is cooled and separated from gas and liquid, and the liquid enters the condensation t...
Embodiment 3
[0033] The method for preparing potassium fluoride in this embodiment uses ammonium fluoride and potassium hydroxide as main raw materials, and specifically comprises the following steps:
[0034] (1) Put 40% ammonium fluoride solution into the reaction kettle, start stirring, and add solid potassium hydroxide in the reaction kettle at a uniform speed. The pH value at the end of the reaction is 8.0. This reaction is an exothermic reaction. After the reaction, the temperature reaches Above 110°C; water vapor is cooled, gas-liquid separated, and the liquid enters the condensation tank; the gas is absorbed into ammonia water through the cooling cycle, enters the storage tank, and is used for standby, and the rest of the gas is emptied;
[0035] (2) After the reaction in the reactor is complete, the liquid flows into the concentration tank, the concentrated water vapor is cooled and separated from gas and liquid, and the liquid enters the condensation tank; the gas is cooled and ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com