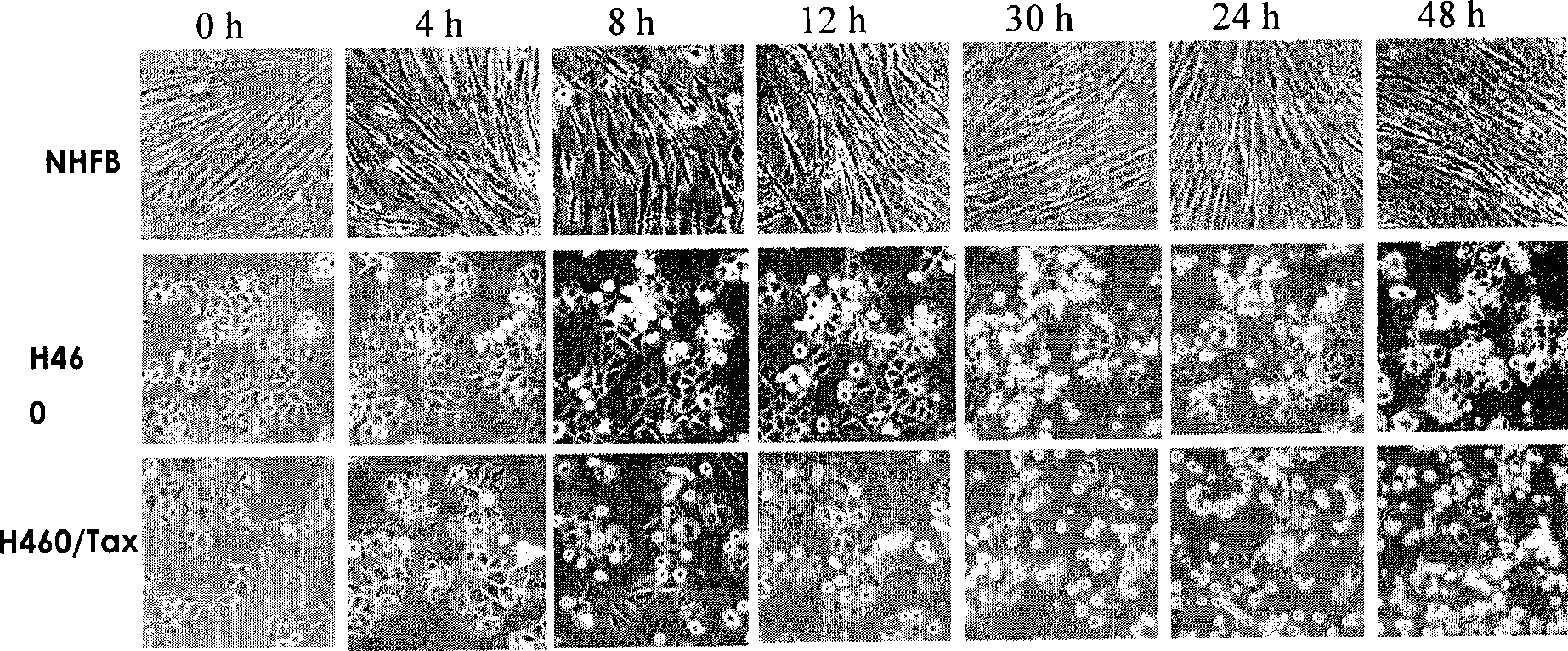
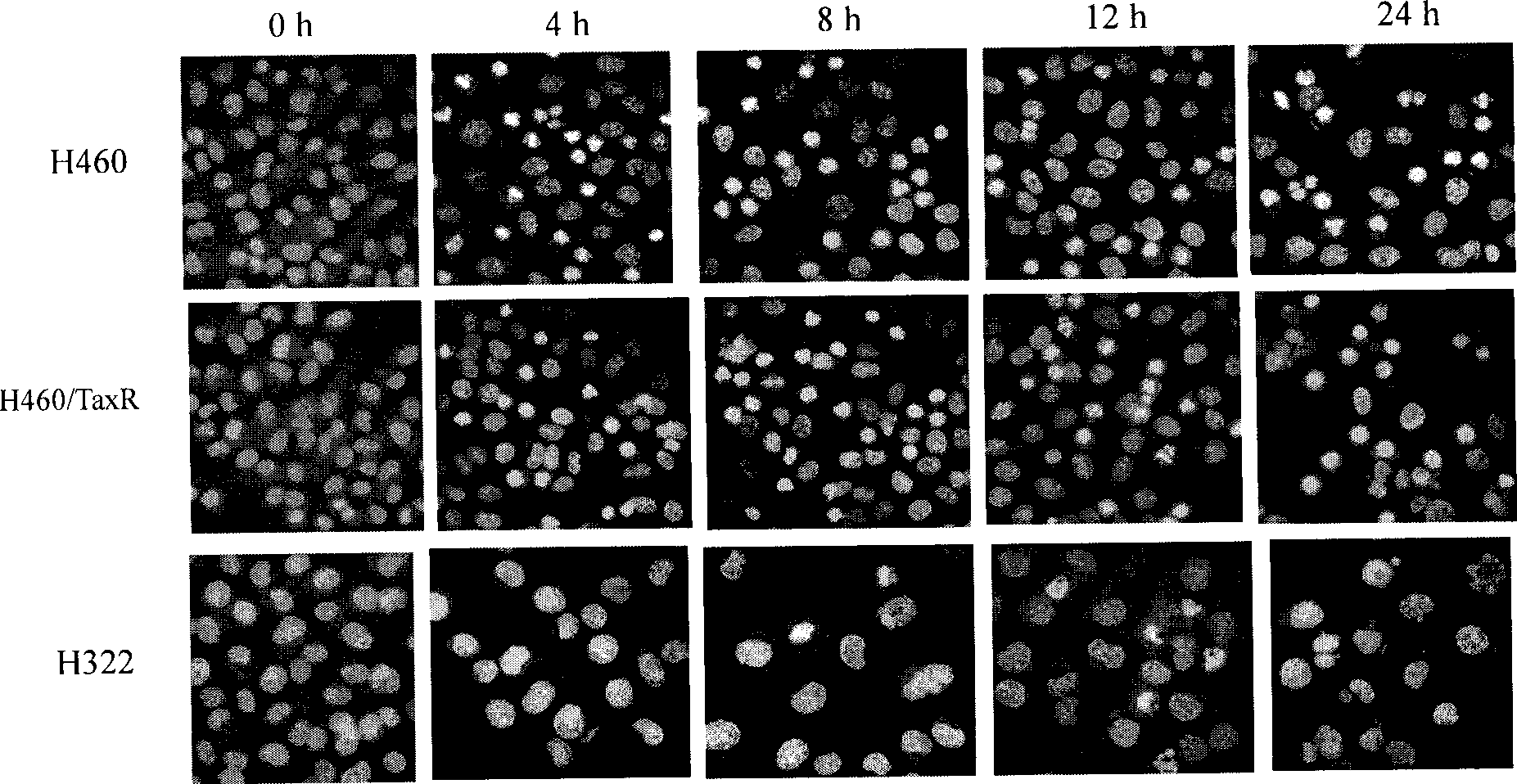
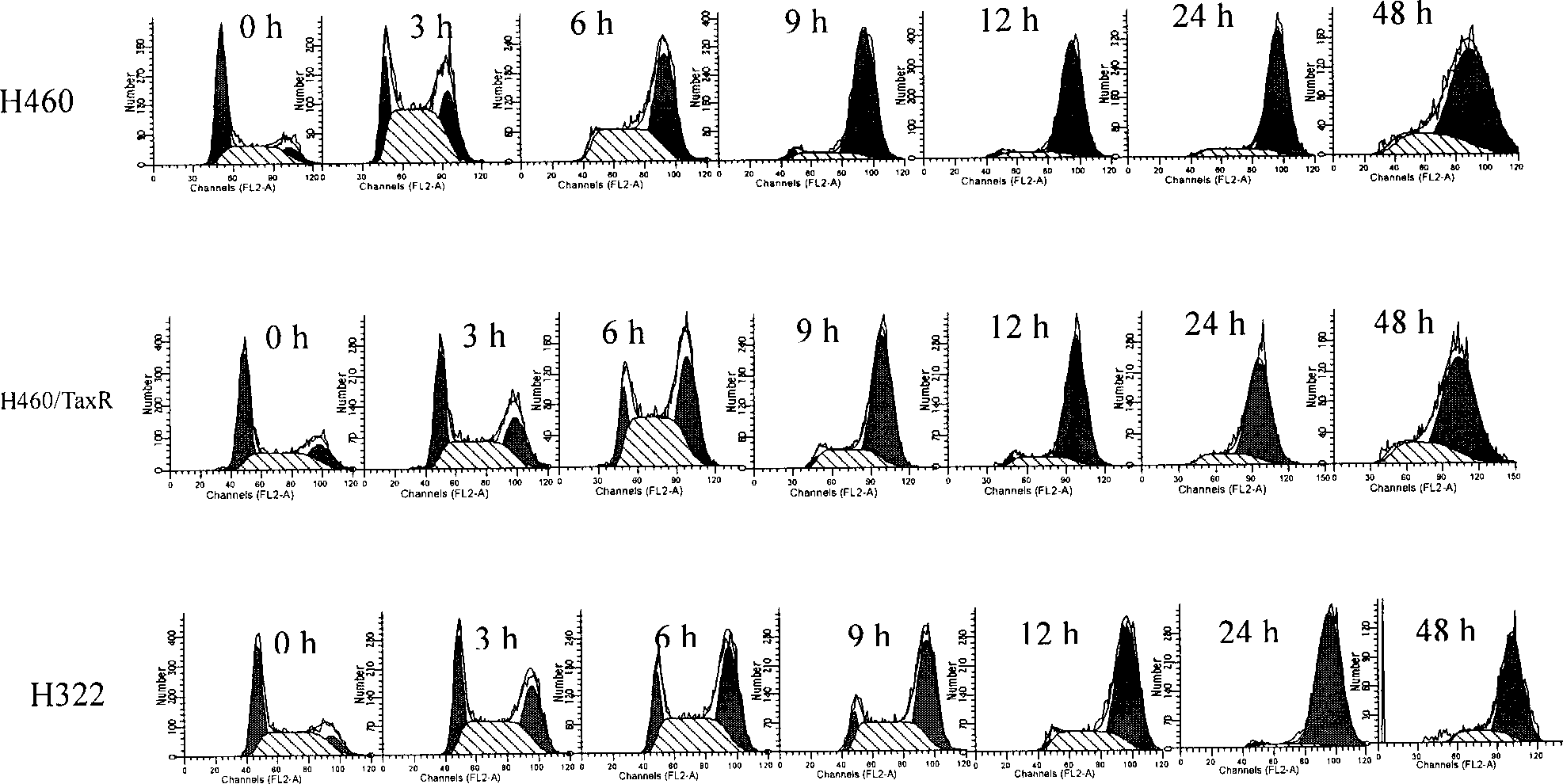
Thiazolidone derivates and application thereof in preparing anti-lung cancer medicine
A thiazolidinone class, the technology of said thiazolidinone, which is applied to novel thiazolidinone derivatives and its application field in the preparation of anti-lung cancer drugs, can solve the toxicity, cannot be used for the treatment of stubborn drug-resistant or recurrent tumors, Problems such as poor outcomes in treating cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of 2-(2-chlorophenylimino)-5-(4-hydroxy-3-methoxybenzylidene)-1,3-thiazol-4-one
[0054]
[0055] by HCl:H 2 The ratio of O (1:4) is dubbed hydrochloric acid solution. Weigh 11.4g (150mmol) of ammonium thiocyanate and dissolve it in 50mL of hydrochloric acid solution, add 10.5mL (100mmol) of 2-chloroaniline, and heat to 85°C under stirring conditions, the mixture becomes clear, after 12 hours of reaction, TLC monitors the reaction, After the reaction, the mixture was cooled to room temperature, and a viscous oily liquid appeared, which was extracted with ethyl acetate, and the extract was washed with 10% hydrochloric acid solution, saturated sodium chloride solution, and water successively, and the solvent was evaporated off the extract under reduced pressure to obtain 2-Chlorophenylthiourea.
[0056] Add 1120mg (6.0mmol) of 2-chlorophenylthiourea and 2479mg (30mmol) of anhydrous sodium acetate into 20mL of ethanol, add 1.28mL (12mmol) of ethyl chloroac...
Embodiment 2
[0060] 2-(2-Chlorophenylimino)-5-(4-methoxybenzylidene)-1,3-thiazol-4-one
[0061]The preparation method is similar to the preparation of the compound of Example 1.
[0062] The product is a yellow powder with a yield of 73.9%; 1 H NMR (400MHz, MeOD): δ (ppm) = 7.53 (s, 1H), 7.38 (d, 1H, J = 8.0), 7.32 (d, 2H, J = 8.4), 7.23 (t, 1H, J = 7.6), 7.09(t, 1H, J=7.6), 6.98(d, 1H, J=7.4), 6.89(d, 2H, J=8.4), 3.72(s, 3H), 1.91, 1.19(ss, 1H ); ESI-MS: m / z 345.6 (M+1) + .
Embodiment 3
[0064] 2-(2-Chlorophenylimino)-5-(2-naphthylidene)-1,3-thiazol-4-one
[0065] The preparation method is similar to the preparation of the compound of Example 1.
[0066] The product is an orange powder with a yield of 99.0%; 1 H NMR (400MHz, MeOD): δ (ppm) = 8.30 (s, 1H), 8.04 (d, 1H, J = 8.1), 7.82 (d, 2H, J = 4.1), 7.49 (m, 3H), 7.37 (dd, 2H, J=7.8), 7.19(t, 1H, J=7.4), 7.06(t, 1H, J=7.6), 6.96(d, 1H, J=7.9), 1.91, 1.18(ss, 1H ); ESI-MS: m / z 365.6 (M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com