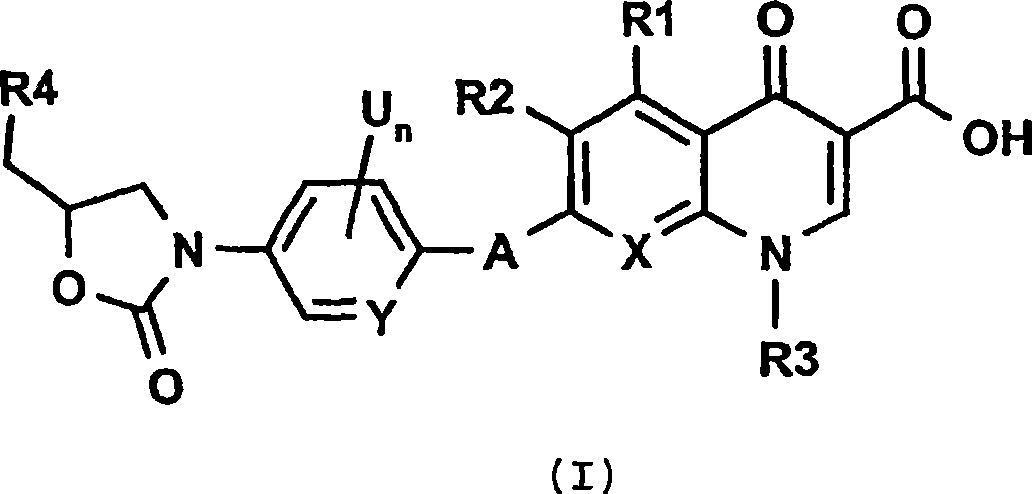
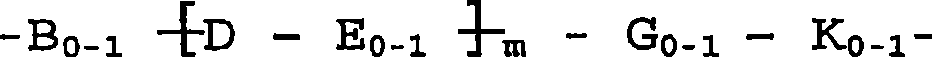Dual action antibiotics
A compound and group technology, applied in the field of dual-action antibiotics, can solve problems such as no bactericidal effect, low safety limit, and emergence of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1: 7-(4-{4-[5-(acetylamino-methyl)-2-oxo-oxazolidin-3-yl]-2-fluoro-phenyl}-piperazin-1-yl )-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid
[0090]
[0091] 7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid boron diacetate (7-chloro-1-cyclopropyl-6-fluoro -4-oxo-1,4-dihydro-quinoline-3-carboxylateboron diacetate) (described in WO8807998; 103mg, 0.25mmol), N-[3-(3-fluoro-4-piperazin-1-yl-benzene base)-2-oxo-oxazolidinone-5-ylmethyl]acetamide (described in J.Med Chem1996,39,673-679 and US5547950; 100mg, 0.3mmol) and N,N'-dimethyl A mixture of p-toluidine (0.054ml, 0.375mmol) was stirred in 0.5ml of 1-methyl-2-pyrrolidone at 120°C for 12 hours. The reaction mixture was poured into water, and the resulting crystals were collected by filtration and purified by silica gel chromatography. Fractions of interest were pooled to yield 38 mg (26%) of beige material.
[0092] C 29 h 29 f 2 N 5 o 6 (581.5812)
[0...
Embodiment 2
[0095] Example 2: 9-(4-{4-[5-(acetylamino-methyl)-2-oxo-oxazolidin-3-yl]-2-fluoro-phenyl}-piperazin-1-yl )-8-fluoro-3-methyl-6-oxo-2,3-dihydro-6H-1-oxa-3a-aza-phenalene-5-carboxylic acid:
[0096]
[0097] 9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido-[1,2,3-des]-1,4-benzoxazine-6- Carboxylic acid (commercially available from Aldrich (47267-0), described in Chem. Pharm. Bull. 1987, 35, 1896-1902, 84 mg; 0.3 mmol), N-[3-(3-fluoro-4-piper Oxazin-1-yl-phenyl)-2-oxo-oxazolidinon-5-ylmethyl]acetamide (described in J. Med Chem 1996, 39, 673-9 and US5547950; 121 mg, 0.36 mmol) and A suspension of DABCO (43.7mg, 0.39mmol) in acetonitrile / water (7ml, 2:1) was refluxed for 12 days. The acetonitrile was removed alkaline and the residue was poured into water. The crystals were collected by filtration and further stirred in methanol (5 ml). The resulting crystals were recrystallized in DMF / water (4:1) to obtain 95 mg of a beige substance (53%).
[0098] C 29 h 29 f 2 N 5 o...
Embodiment 3
[0101] Example 3: 7-((3R,S)-3-{4-[(5S)-5-(acetylamino-methyl)-2-oxo-oxazolidin-3-yl]-2-fluoro- Phenylcarbamoyl}-piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid
[0102]
[0103] 2([(5S)-5-(Acetamidomethyl)-2-oxo-oxazolidin-3-yl]-2-fluoro-phenylcarbamoyl)-piperazine-1,4-dicarboxylic acid Di-tert-butyl ester
[0104] Add 0.210ml of phosphorus oxychloride (phosphoroxychloride) at -15°C to 0.4g of N[(5S)-3-(4-amino-3-fluoro-phenyl)-2-oxo-oxazolidine in 10ml of pyridine -5-ylmethyl]acetamide (1.5mmol) and 0.545g piperazine-1,2,4-tricarboxylic acid 1-4-di-tert-butyl ester (1.65mmol). The reaction was monitored by TLC. The reaction mixture was poured onto ice, diluted with dichloromethane, the organic layer was washed with water and brine, dried over magnesium sulfate, filtered and evaporated. The residue was purified by chromatography using dichloromethane / methanol 95 / 5 as eluent to give a colorless foam.
[0105] Yield: 0.390 g, 45%, C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com