Alpha-glucosidase, gene thereof, preparation method, vector and host cell
A technology of glucosidase and recombinant host cells, applied in botany equipment and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of low enzyme activity, limited expression, limited application, etc., to achieve Effects of high-efficiency expression, strong sucrose hydrolysis function, high temperature resistance and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
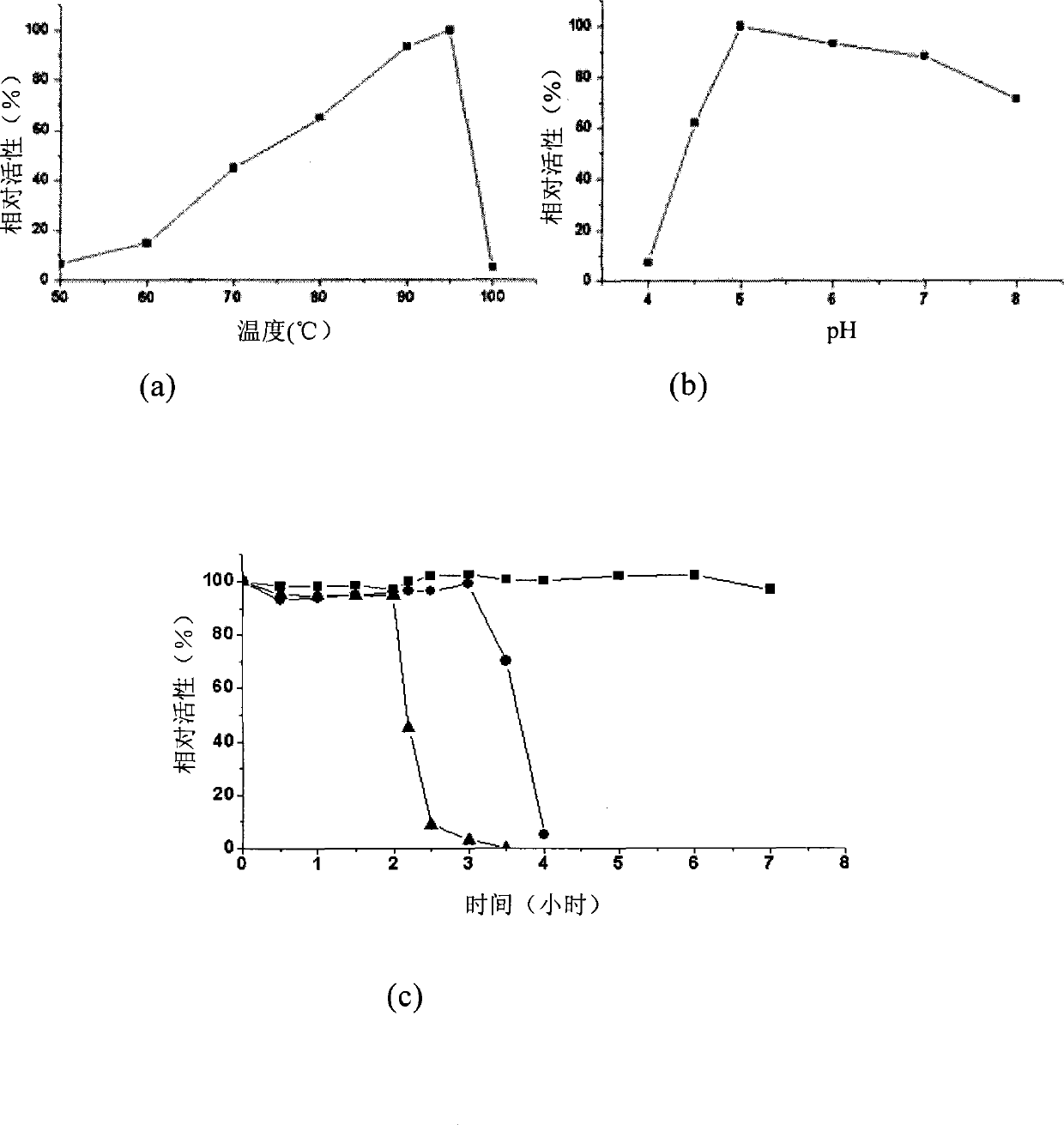
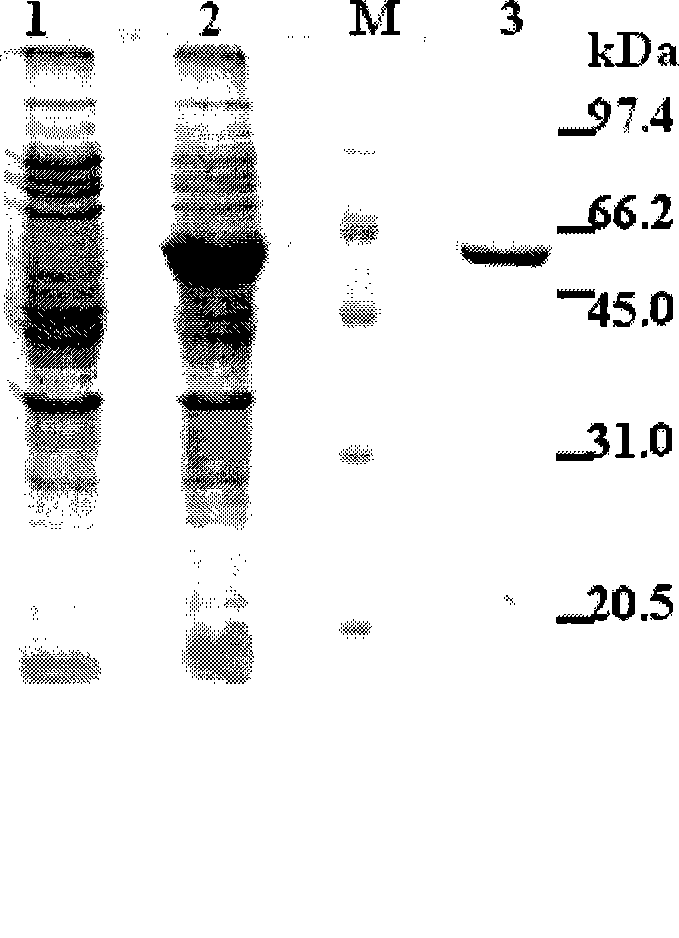
Image
Examples
Embodiment 1
[0038] Embodiment 1. Containing the construction of the recombinant cloning vector pGEM-TGlu plasmid of the sequence shown in SEQ ID: NO.1 and transformed strain DH5α (pGEM-TGlu)
[0039] 2L of water samples were collected from Hamazui Hot Spring (pH8, temperature 78°C) in Tengchong, Yunnan, and the bacteria in the water samples were collected by filtration through a 0.22 μm microporous membrane (Milipore Company), and washed with STE buffer (0.1M NaCl; 10mM Tris -HCl pH8.0; 1mM EDTA pH8.0) to wash the bacteria on the filter membrane, and centrifuge at 4000rpm for 10 minutes to obtain a fresh wet weight of about 0.5g of the bacteria. UltraClean for bacteria sediment TM The total DNA solution (100 ul, 0.4 μg / μl) was extracted by Soil DNAKit, and the absorbance ratio of the total DNA solution was determined: A260 / A280=1.947, A260 / A230=2.15. Take 0.2 μl of the above total DNA solution as a template, and design primers according to the upstream and downstream sequence information...
Embodiment 2
[0042] The construction of embodiment 2.α-glucosidase expression vector and transformed bacterial strain:
[0043] Both the pGEM-TGlu plasmid and the plasmid pET28a were digested with Nhe I and HindIII (50 μl digestion system contains 20 μg of plasmid, 20 U of each restriction enzyme, digested at 37°C for 4 hours), and the gel was cut by agarose electrophoresis After recovering the target insert and pET28a digested fragment (recovered in 20 μl deionized water respectively), take the two digestion products and connect (5 μl ligation system contains 1 μl pET28a digested fragment, 3 μl target insert fragment, 1 μl Promega T4 DNA ligation Enzyme, ligated overnight at 16°C) to construct a pETGlu recombinant expression vector, take 2 μl of the ligated product for electric shock (voltage 1.8KV, capacitance 25μF, electric shock time 3ms) to transform Escherichia coli BL21 (DE3) to obtain a recombinant expression vector containing SEQ ID: NO.1 The transformed strain BL21(DE3)(pETGlu) e...
Embodiment 3
[0044] Example 3. Preparation of α-glucosidase gene mutants and construction of transformed strains thereof
[0045]Using Stratagene's site-directed mutagenesis kit (QuikChange site-directed mutagenesis kit), using the expression vector pETGlu of Example 2 as a template, referring to the instructions, by introducing mutant bases into the primers and performing PCR amplification, SEQ ID: NO. 1 Perform the following 4 site-directed mutations: (1) mutation of A at position 211 to G; (2) mutation of G at position 272 to A; (3) mutation of C at position 1546 to T, and mutation of T at position 1571 to C (4) A mutation at position 211 to G, G at position 272 to A, C at position 1546 to T, and T at position 1571 to C. After the resulting mutant products were digested with Dpn I (20 μl system containing 15 μl PCR product and 5U Dpn I, digested at 37° C. for 2 hours), each 2 μl was electrotransformed (the same conditions as in Example 2) to E. coli XL1-Blue. state cells, obtain vector...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com