Electrochemistry method for simultaneously producing sodium chlorate and alkaline peroxide
A technology of hydrogen peroxide and sodium chlorate, which is applied in the direction of electrolysis process, electrolysis components, cells, etc., can solve the problems of increasing operating costs, achieve the effect of reducing operating costs, reducing production costs, and improving overall utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
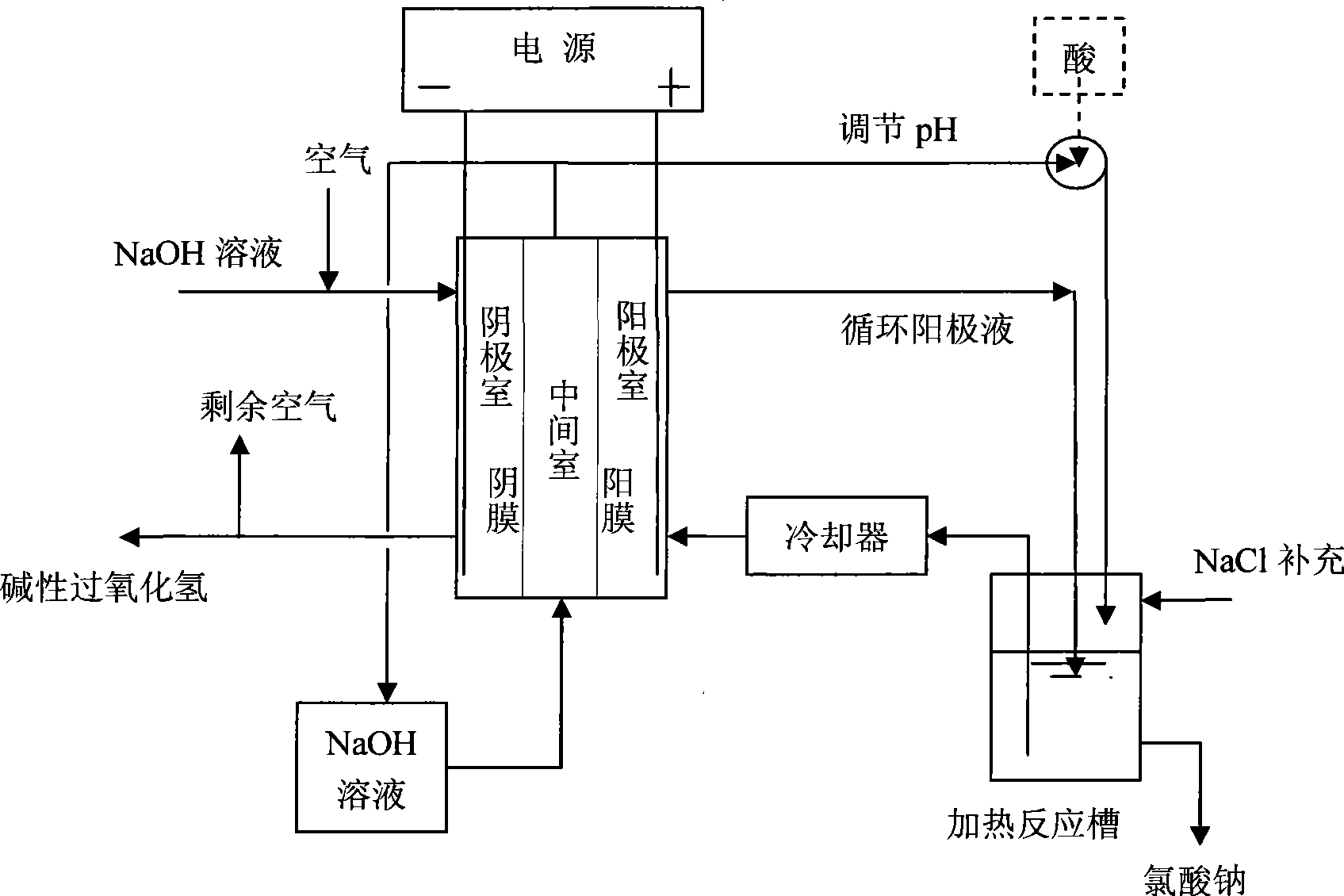
[0025] use as figure 1 Shown is the experimental setup for the electrochemical process to prepare alkaline hydrogen peroxide and sodium chlorate. The specific test conditions are as follows: the cathode material is carbon black / polytetrafluoroethylene coated composite graphite particles, the nickel cathode plate (80*70mm), the anode is a titanium-coated lead dioxide DSA electrode (80*70mm), and the apparent area of the electrode is 30cm 2 , the anion exchange membrane is a polyethylene heterogeneous membrane, and the cation exchange membrane is a polyethylene heterogeneous membrane. The anolyte is 3.8M NaCl, and the residence time in the anode chamber is 21s; the catholyte is 2M NaOH, and the residence time is 7min; The concentration is 1M, and the residence time is 100s. The temperature of the anolyte in the electrolytic cell is 30°C and that of the catholyte is 35°C. The current density is 1.2kA / m 2 , the average terminal voltage is 4.3V. The temperature of the heati...
Embodiment 2
[0027] use as figure 1 Shown is the experimental setup for the electrochemical process to prepare alkaline hydrogen peroxide and sodium chlorate. The specific test conditions are as follows: the cathode material is carbon black / polytetrafluoroethylene coated composite graphite particles, the stainless steel cathode plate (80*70mm), the anode is a titanium-coated lead dioxide DSA electrode (80*70mm), and the apparent area of the electrode is 30cm 2 , the anion exchange membrane is a polysulfone heterogeneous membrane, and the cation exchange membrane is a perfluorosulfonic acid membrane. Anolyte is 2.8M NaCl and 2.42M NaClO 3 , the residence time in the anode chamber is 8s; the catholyte is 2M NaOH, and the residence time is 84s; The time is 10s. The temperature of the anolyte in the electrolytic cell is 30°C and that of the catholyte is 35°C. The current density is 1.2kA / m 2, the average terminal voltage is 4.1V. The temperature of the heating reaction tank is 70° C.,...
Embodiment 3
[0029] use as figure 1 Shown is the experimental setup for the electrochemical process to prepare alkaline hydrogen peroxide and sodium chlorate. The specific test conditions are as follows: the cathode material is carbon felt, the stainless steel cathode plate (80*70mm), the anode is a titanium-coated ruthenium Ru / Ti electrode (80*70mm), and the apparent area of the electrode is 30cm 2 , the anion exchange membrane is a polysulfone heterogeneous membrane, and the cation exchange membrane is a perfluorosulfonic acid membrane. Anolyte is 3.8M NaCl and 0.25M NaClO 3 , the first residence time in the anode chamber is 42s; the catholyte is 4M NaOH, the residence time is 42s, the air is passed into the cathode chamber after the alkali washing bottle and the water washing bottle, and the residence time is 0.2s; the NaOH solution concentration in the middle chamber is 2M, The dwell time is 5s. The temperature of the anolyte in the electrolytic cell is 50°C, and that of the catho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com