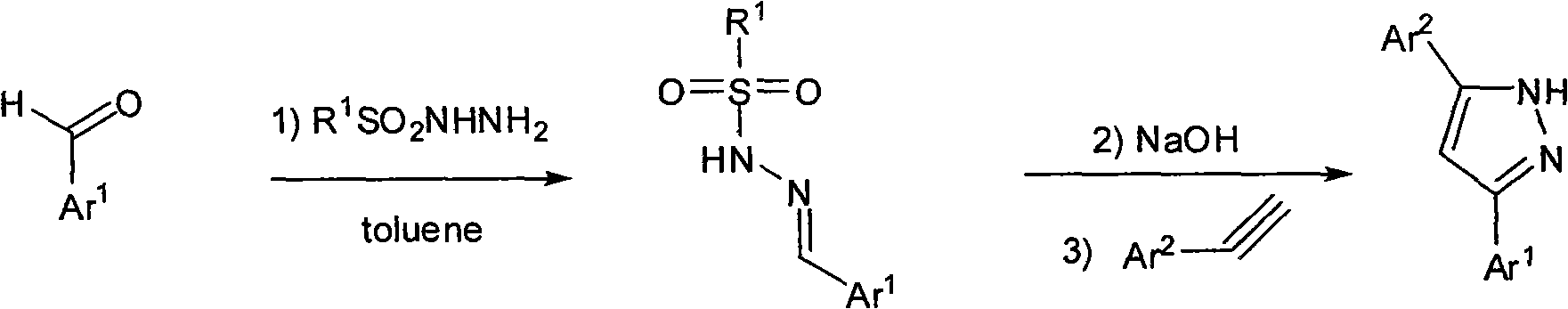
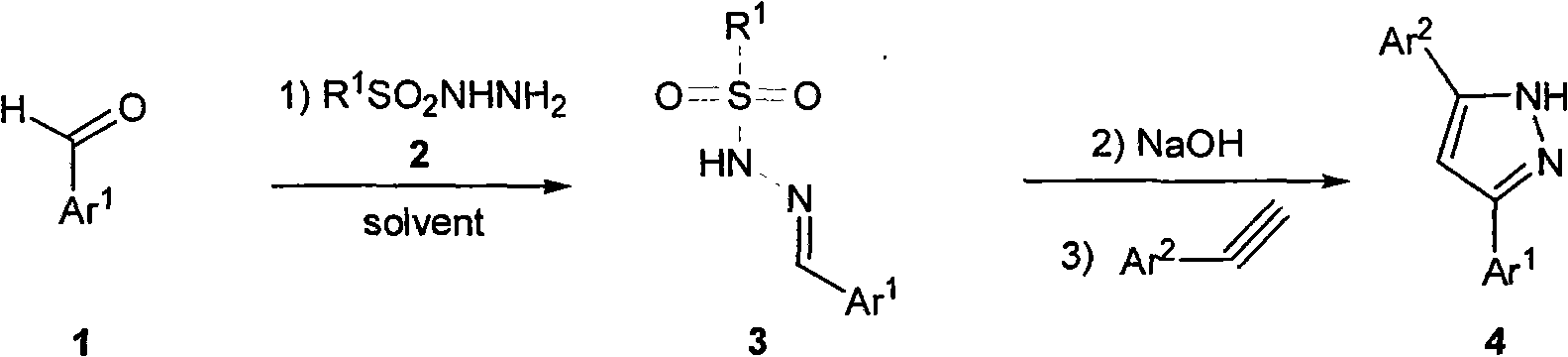
Method for synthesizing 3,5-disubstituted pyrazole
A synthesis method and a secondary substitution technology, applied in 3 fields, can solve the problems of difficult separation and purification, poor economy, high synthesis cost, etc., and achieve the effects of simple synthesis method, reduced dosage, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
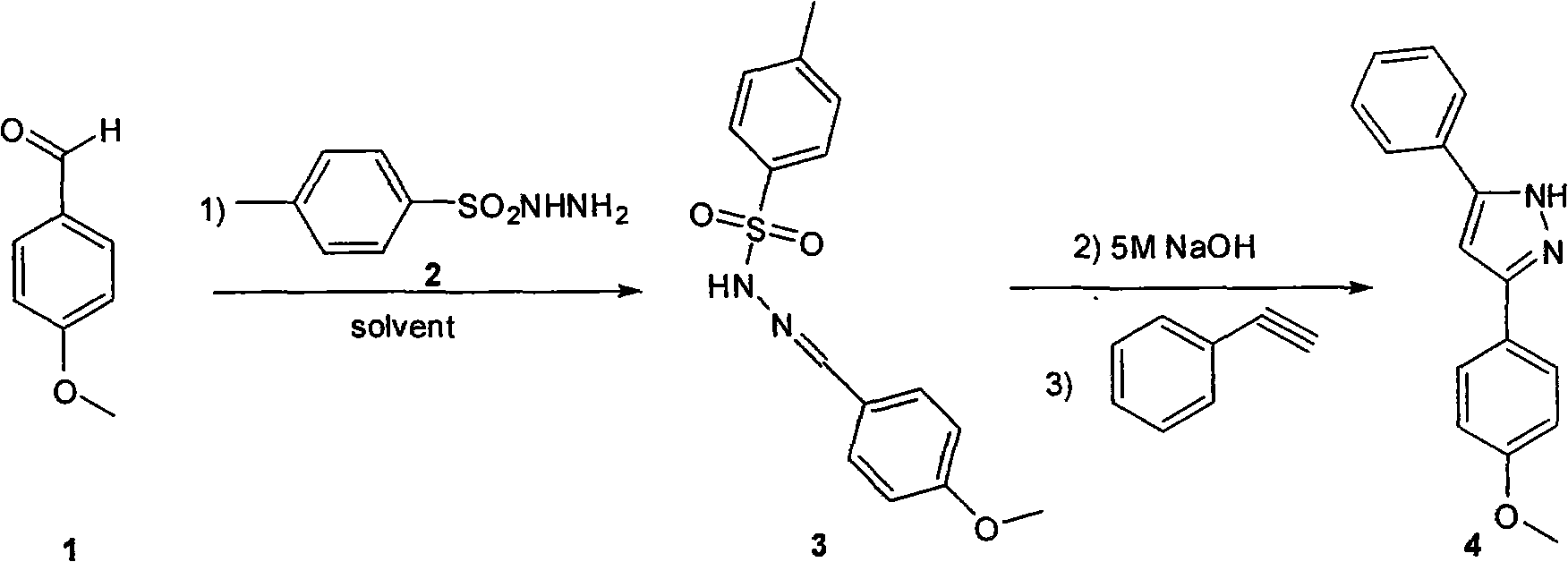
[0030] In the first step, p-methoxybenzaldehyde (136mg, 1mmol) was dissolved in 6mL of toluene, then p-toluenesulfonyl hydrazide (186mg, 1mmol) was added, and stirred at room temperature for 3 hours; in the second step, 5mol / L of Sodium hydroxide aqueous solution (0.2mL, 1mmol) was stirred at room temperature for 20 minutes; in the third step, phenylacetylene (153mg, 1.5mmol) was added, and the temperature was raised to 45°C, and the reaction was completed after 12 hours. After cooling to room temperature, the solvent and a little water were evaporated by rotary evaporation, and the organic phase was eluted with ethyl acetate. LCMS showed that the purity of the crude product was 85%.
[0031] The crude product above is separated by preparative plate, obtains 220mg product, proton nuclear magnetic resonance spectrum [ 1 H NMR (400MHz, CDCl 3 ): δ=7.72(d, 2H), 7.63(d, 2H), 7.38(t, 2H), 7.32(t, 1H), 6.90(d, 2H), 6.75(s, 1H), 3.82(s, 3H)] shows the correct structure;...
Embodiment 2
[0033]
[0034] In the first step, 5-bromopyridine-3-carbaldehyde (186 mg, 1 mmol) was dissolved in 6 mL of toluene, then p-toluenesulfonyl hydrazide (186 mg, 1 mmol) was added, and stirred at room temperature for 3 hours; in the second step, 5 mol / L of sodium hydroxide aqueous solution (0.2mL, 1mmol) was stirred at room temperature for 20 minutes; in the third step, phenylacetylene (153mg, 1.5mmol) was added, the temperature was raised to 45°C, and the reaction was completed after 12 hours. After cooling to room temperature, the solvent and a little water were evaporated by rotary evaporation, and the organic phase was eluted with ethyl acetate. LCMS showed that the purity of the crude product was 96%.
[0035] The crude product above is separated by preparative plate, obtains 260mg product, proton nuclear magnetic resonance spectrum [ 1 H NMR (400MHz, CDCl 3 ): δ=9.15(s, 1H), 8.21(s, 1H), 8.19(s, 1H), 7.72(d, 2H), 7.38(t, 2H), 7.32(t, 1H), = 6.75(s , 1H)] shows the cor...
Embodiment 3
[0037]
[0038] In the first step, dissolve p-benzaldehyde (106 mg, 1 mmol) in 6 mL of toluene, then add p-toluenesulfonyl hydrazide (186 mg, 1 mmol), and stir at room temperature for 3 hours; in the second step, add 5 mol / L of sodium hydroxide Aqueous solution (0.2mL, 1mmol) was stirred at room temperature for 20 minutes; in the third step, p-methylphenylacetylene (174mg, 1.5mmol) was added, the temperature was raised to 45°C, and the reaction was completed after 12 hours. After cooling to room temperature, the solvent and a little water were evaporated by rotary evaporation, and the organic phase was eluted with ethyl acetate. LCMS showed that the purity of the crude product was 91%.
[0039] The crude product above is separated by preparative plate, obtains 210mg product, proton nuclear magnetic resonance spectrum [ 1 H NMR (400MHz, CDCl 3): δ=7.72(d, 2H), 7.63(d, 2H), 7.32(t, 1H), 7.18(d, 2H), 6.90(d, 2H), 6.75(s, 1H), 2.18(s, 3H)] shows the correct structure; m / z=235...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com