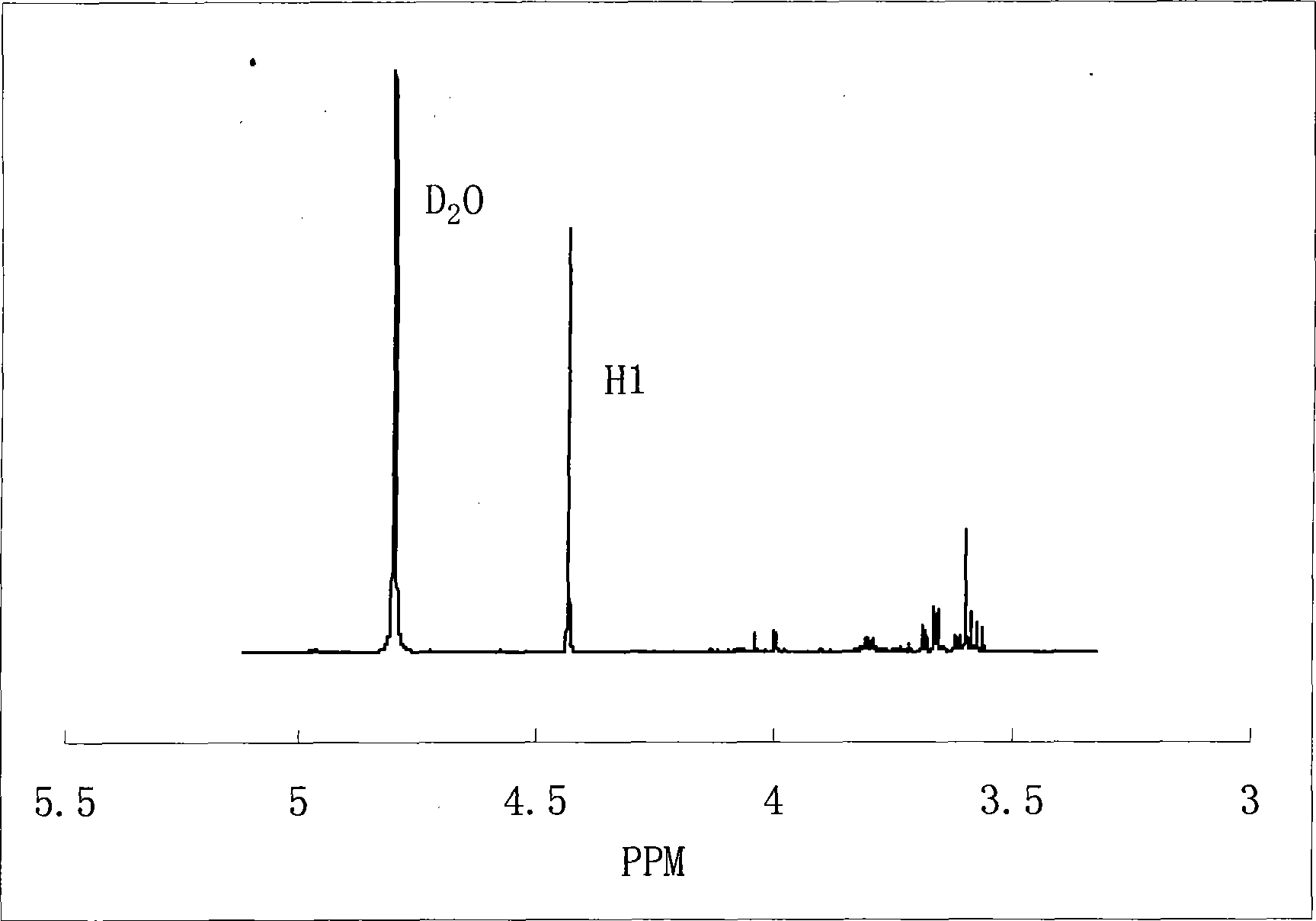
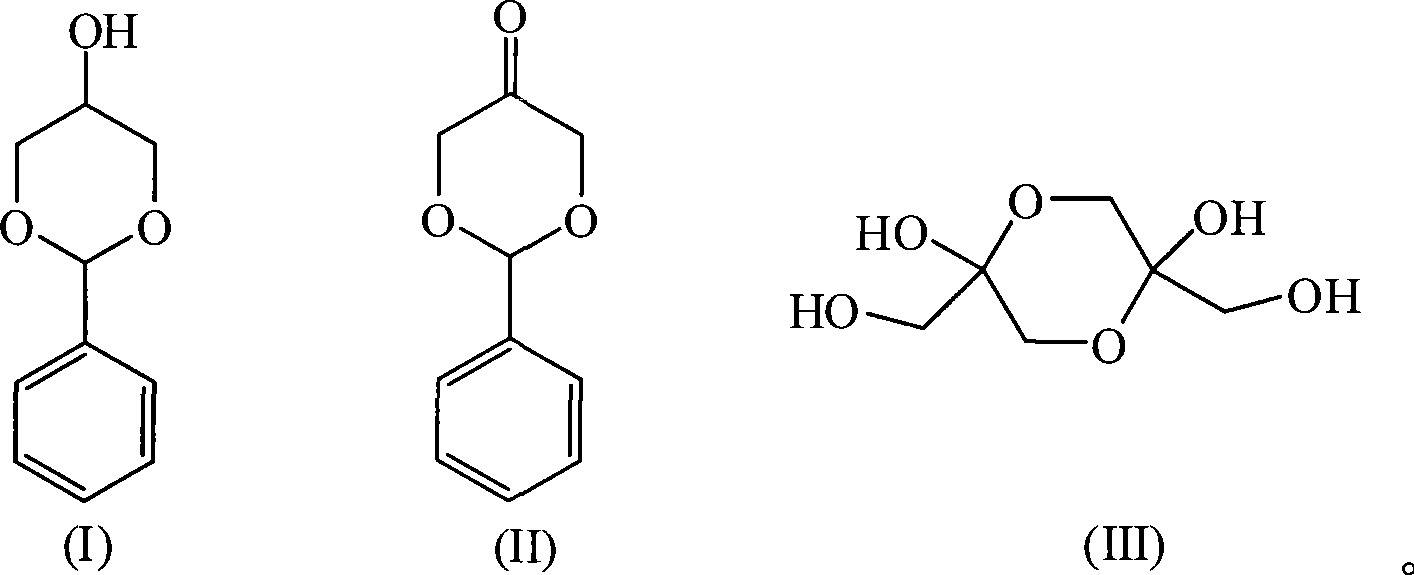
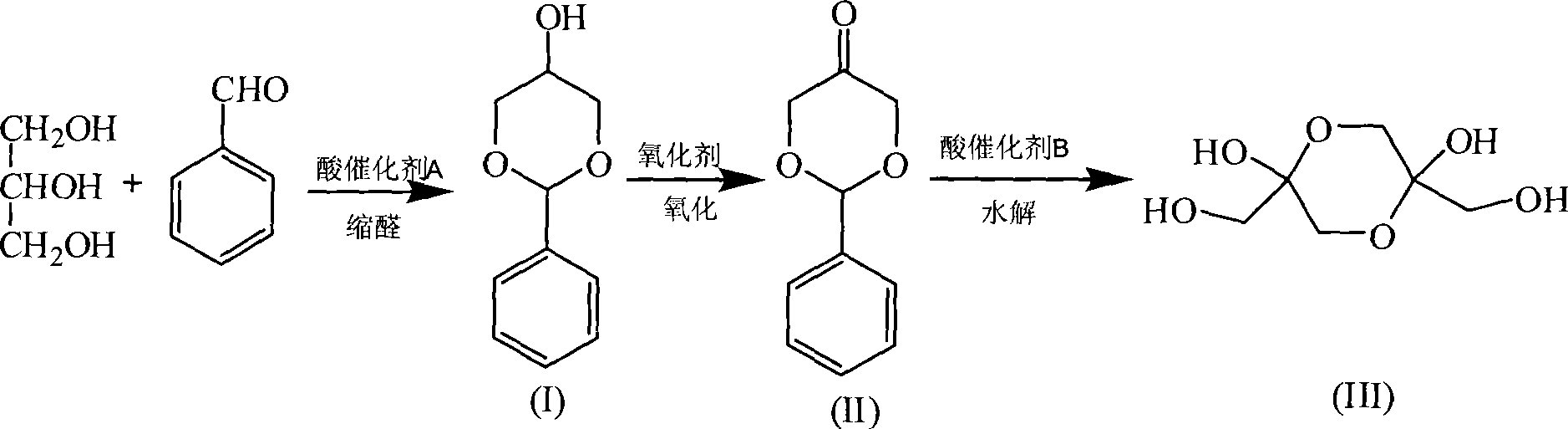
Novel method for preparing 1,3-dihydroxy acetone from glycerol
A technology of dihydroxyacetone and glycerin, which is applied in the first field, can solve the problems of long reaction time and poor selectivity, and achieve the effects of large market competitiveness, simple process, high selectivity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Step (1): Add 0.6 g of p-toluene sulfonic acid, 58.0 g of glycerin, 61.0 g of benzaldehyde, and 100 ml of benzene into a 250 ml round bottom flask equipped with a condenser and a water separator, heat and stir, and condense and reflux. The reaction temperature was controlled at 130°C, and the reaction time was 2 hours. The reaction solution was washed with water, dried with anhydrous potassium carbonate, and distilled under reduced pressure (to recover benzene), and then crystallized in a mixed solution of benzene and petroleum ether (volume ratio 1:1) at -10°C to obtain 12.4 g of glycerol benzaldehyde acetal ether. The yield was 12.3%. The recovered mother liquor of benzene and acetal crystallization is kept for use.
[0043] Step (2): Add 1 mol of chromium trioxide slowly to 2 mol of pyridine, and control the reaction temperature below 40°C. Under constant stirring, yellow pyridine chromium oxide precipitates will be produced. Continue to stir, and the yellow precipitate ...
Embodiment 2
[0046] Step (1): Add 1ml 98% sulfuric acid, 58g glycerin, 61g benzaldehyde (with an excess of 6% benzaldehyde) and 100ml acetal crystal mother liquor in a 250ml round bottom flask equipped with a condenser and a water separator, and heat and stir. , Condensate to reflux, reaction temperature 130°C, reaction time 6 hours. After the completion of the reaction, the reaction solution was washed with water, dried with anhydrous potassium carbonate, distilled under reduced pressure (recovered benzene) and crystallized in a mixed solution of benzene and petroleum ether (1:1) at -10°C to obtain glycerol benzaldehyde acetal ether 21.6 g, the yield was 21.4%. The recovered benzene and crystallization mother liquor are kept for use.
[0047] Step (2): Quickly add 100g of chromium trioxide to 6mol / L hydrochloric acid (containing 1.1molHCl) (adding while stirring), after 5 minutes, cool the homogeneous solution to 0°C to obtain a reddish brown liquid, which is filtered and removed under normal...
Embodiment 3
[0050] Step (1): In a 250ml round bottom flask equipped with a condenser and a water trap, add 6g of D072 strong acid cation exchange resin, 58.0g of glycerin, 61.0g of benzaldehyde (with an excess of 6% benzaldehyde), and 100ml of benzene. Heating and stirring, condensing and refluxing, the water produced by the reaction and the azeotroping agent benzene are azeotropically evaporated. The reaction temperature was controlled at 110°C, and the reaction time was 6 hours. After the completion of the reaction, the reaction solution was washed with water, dried with anhydrous potassium carbonate, and distilled under reduced pressure (to recover benzene). Then, 18.0 g of glycerol benzaldehyde acetal ether was obtained in a mixed solution of benzene and petroleum ether (1:1) at -10°C. The yield is 17.9%. The recovered mother liquor of benzene and acetal crystallization is kept for use.
[0051] Step (2): Add 50 g (0.5 mol) of chromium trioxide in a beaker containing 20 mL of water. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com