Method for preparing pentaerythritol bis-phosphite antioxidant
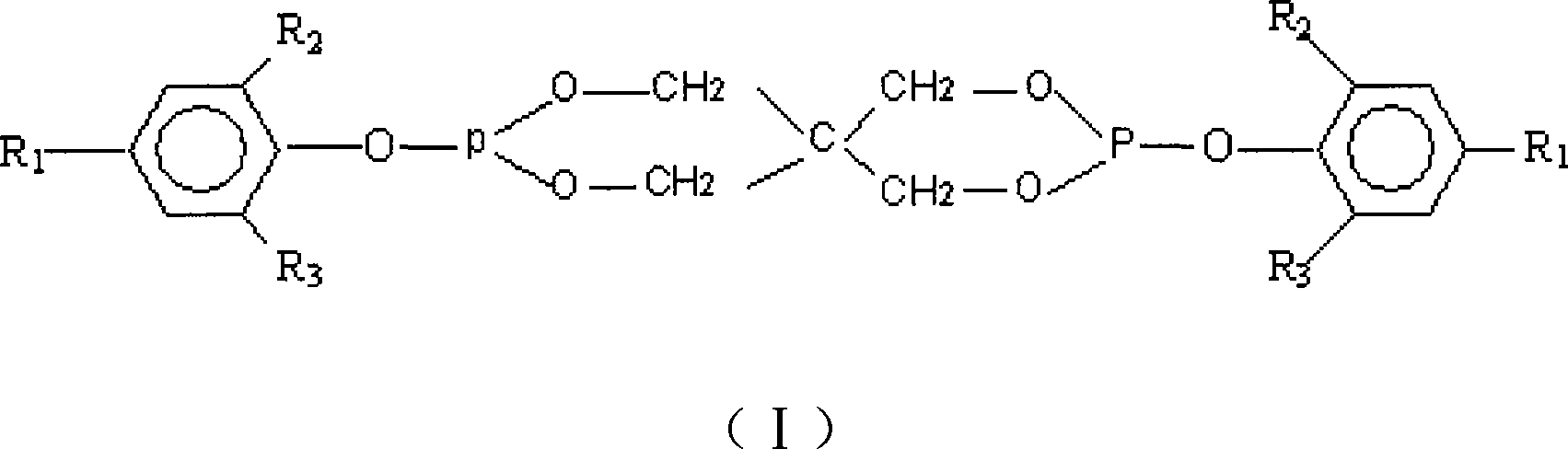
A pentaerythritol bisphosphite and pentaerythritol technology are applied in the field of preparation of pentaerythritol bisphosphite antioxidants, which can solve the problems of complex purification process of intermediate products, unsuitable for industrialized production, increased cost and the like, and achieve good high-temperature antioxidant performance , Good color protection ability, the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
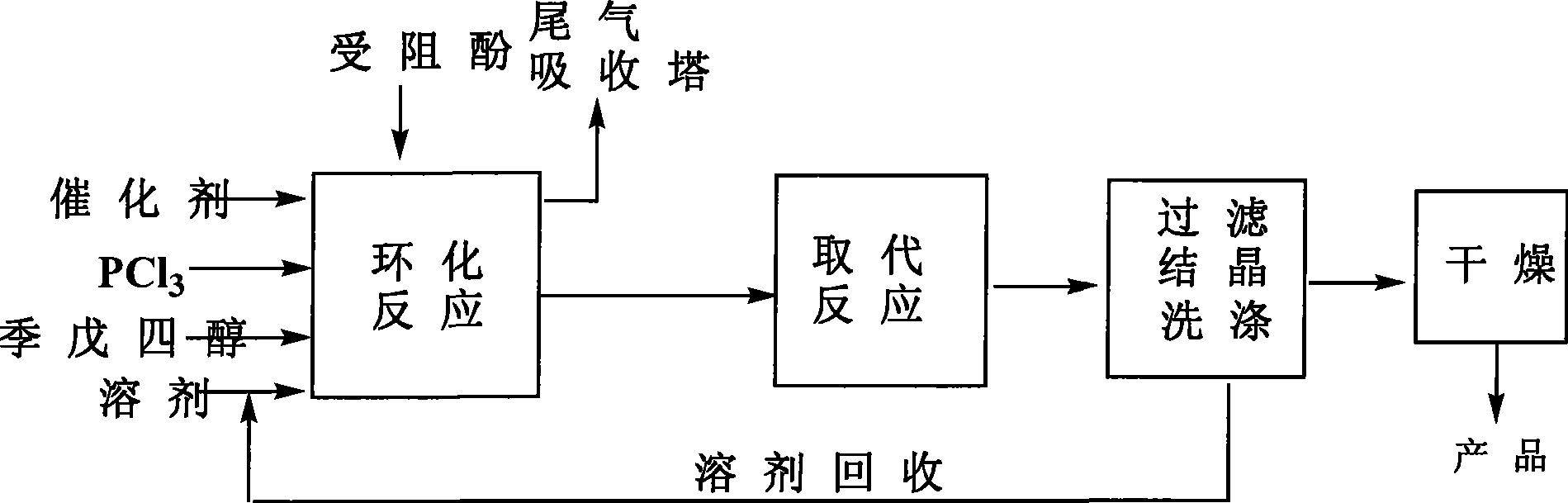
[0070] Add 1 mol of pentaerythritol, 2.5 mol of hindered phenol, and 1000 mL of toluene solvent into a 5 L four-necked flask, and add 0.5 g of pyridine as a cyclization catalyst. Access to N 2 , start stirring, and heat in a water bath. When the temperature is constant at 50°C, start to add PCl dropwise 3 . After the dropwise addition, slowly heat to 80°C to make the cyclization reaction proceed smoothly. After reacting for 8h, adopt 31 P NMR Analyze and monitor the reaction process until the reaction of pentaerythritol and phosphorus trichloride is complete at the same time, add 1000mL triethylamine as a replacement catalyst, heat to 110°C, and keep for 10 hours. The obtained material was filtered while hot, added 3256mL of isopropanol, heated to 70°C and stirred, filtered, then added with 1321mL of n-hexane, stirred and filtered, and the obtained filter cake was dried at 90°C to obtain 325g of the product with a yield of 51.4%.
Embodiment 2
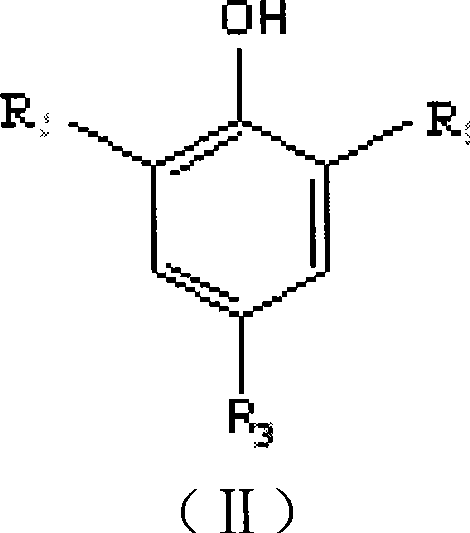
[0072] Add 1mol of pentaerythritol and 1000mL of toluene solvent into a 5L four-necked flask, and add 0.65g of weakly basic anion resin as a cyclization catalyst. Access to N 2 , start stirring, and heat in a water bath. When the temperature is constant at 50°C, start to add PCl dropwise 3 . After the dropwise addition, slowly heat to 80°C to make the cyclization reaction proceed smoothly. After reacting for 5h, adopt 31 P NMR Analyze and monitor the reaction process until the simultaneous reaction of pentaerythritol and phosphorus trichloride is complete. Then add 2.2mol of 2,6-di-tert-butyl-p-cresol, and at the same time add 800mL of tributylamine as a replacement catalyst, heat to 110°C and keep for 10 hours, filter the obtained material while it is hot, then add toluene and heat to boiling, room temperature After crystallization and filtration, the filter cake was washed by adding n-heptane, and after filtration, the resulting filter cake was dried to obtain 372 g of ...
Embodiment 3
[0074] Add 1 mol of pentaerythritol and 1000 mL of toluene solvent into a 5 L four-necked flask, and add 0.5 g of pyridine as a cyclization catalyst. Access to N 2 , start stirring, and heat in a water bath. When the temperature is constant at 50°C, start to add PCl dropwise 3 . After the dropwise addition, slowly heat to 80°C to make the cyclization reaction proceed smoothly. After reacting for 8h, adopt 31 P NMR Analyze and monitor the reaction process until the simultaneous reaction of pentaerythritol and phosphorus trichloride is complete. Then add 4mol of 2,4-di-tert-butylphenol, and at the same time add triethylamine as a replacement catalyst, heat to 120°C, keep for 10 hours, filter the obtained material while it is hot, add xylene and heat to boiling, crystallize at room temperature, filter, The filter cake was washed by adding n-heptane, and after filtration, the resulting filter cake was dried to obtain 384 g of product, with a yield of 63.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com