Method for preparing double effect oxygen electrode catalyst
A double-effect oxygen electrode and catalyst technology, applied in battery electrodes, electrochemical generators, components of fuel cells, etc., can solve the problems of complicated procedures and cumbersome processes, and achieve simple operation, low preparation cost, and easy preparation in the preparation process. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
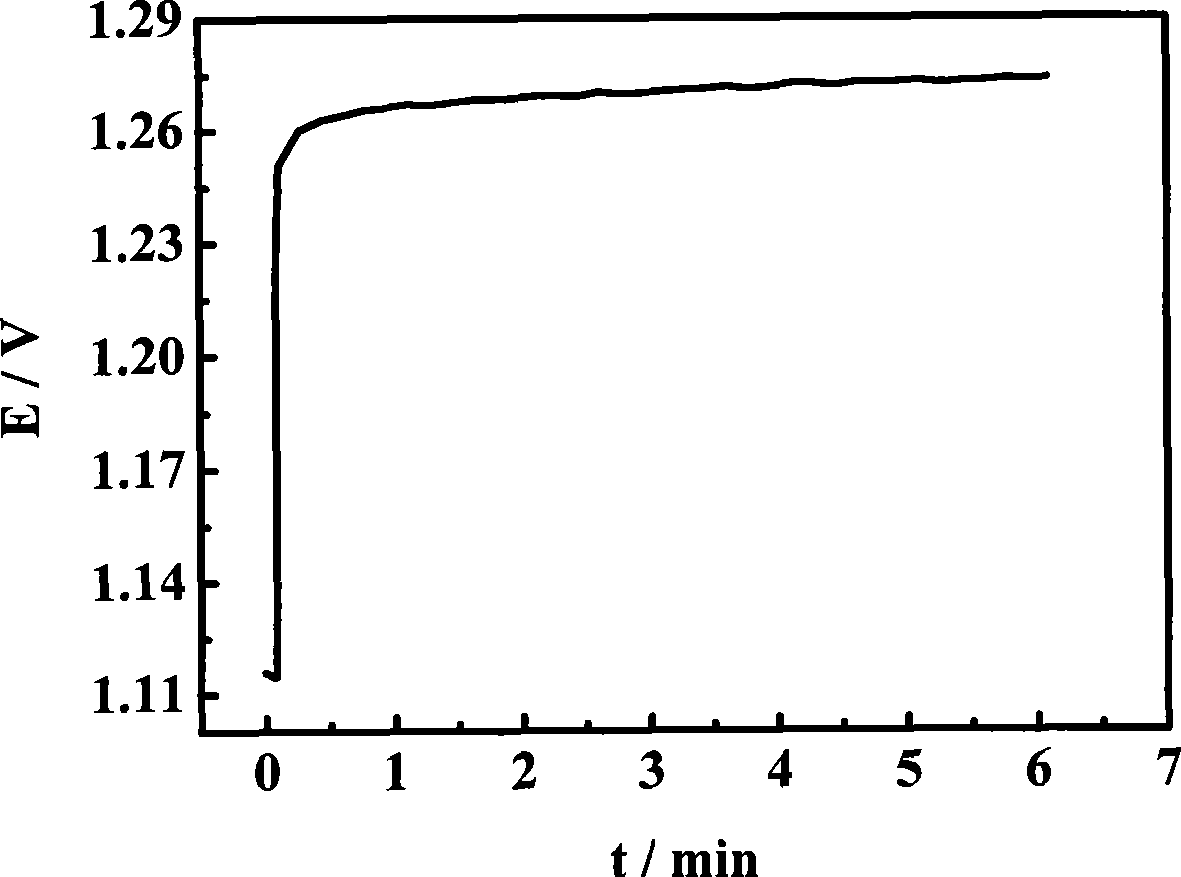
[0022] Mix 4mL of ethanol and 6mL of isopropanol to prepare 10mL of organic solvent, then add 15 mg of H to the organic solvent 2 IrCl 6 and 8 mg H 2 PtCl 6 , ultrasonically disperse the mixed solution for 30 minutes to make a mixed solution; pipette 20 μL of the mixed solution per square centimeter of substrate area, and drop it on the surface of the titanium plate. After the organic solvent evaporates, put the titanium plate into a 95°C vacuum oven in vacuum drying for 10 minutes; put it into a tube furnace and sinter it at 350°C for 20 minutes to make H 2 IrCl 6 、H 2 PtCl 6 Thermally decompose to form metal and metal oxide coatings; take out the air and cool to room temperature, repeat the above steps until the required loading capacity is 2mg / cm 2 ;Finally placed in a tube furnace and sintered at 480°C for 0.5 hours, the prepared IrO 2 / Pt catalyst, performance such as figure 1 shown.
Embodiment 2
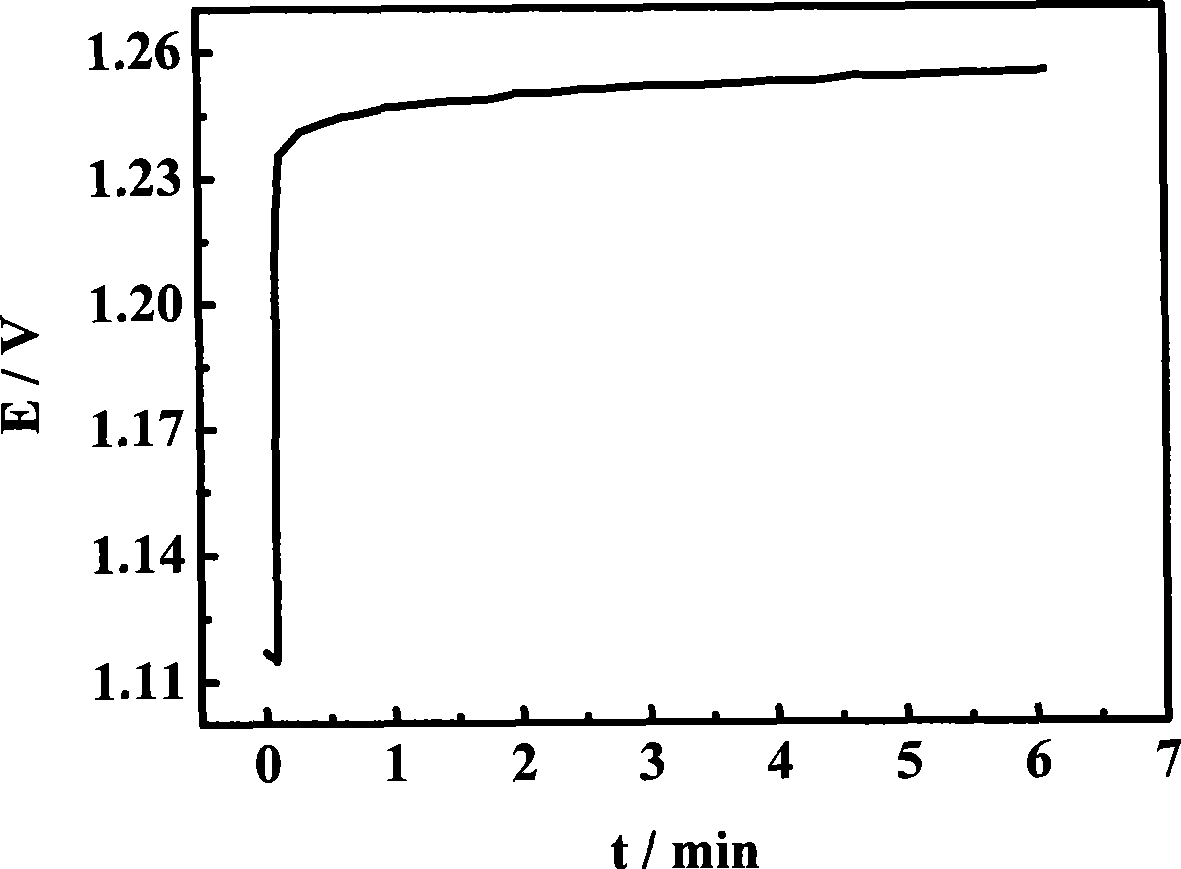
[0024] Mix 5 mL of ethanol and 5 mL of isopropanol to prepare 10 mL of organic solvent, then add 10 mg of H 2 IrCl 6 and 6 mg H 2 PtCl 6 , ultrasonically disperse the mixed solution for 20 minutes to make a mixed solution; pipette 50 μL of the mixed solution per square centimeter of substrate area, and drop it on the surface of the titanium plate. After the organic solvent evaporates, put the titanium plate into a 75°C vacuum oven in vacuum drying for 5 minutes; put it into a tube furnace and sinter at 450°C for 10 minutes to make H 2 IrCl 6 、H 2 PtCl 6 Thermally decompose to form metal and metal oxide coatings; take out the air and cool to room temperature, repeat the above steps until the required loading capacity is 5mg / cm 2 ;Finally placed in a tube furnace and sintered at 450°C for 1 hour to prepare IrO 2 / Pt catalyst, performance such as figure 2 shown.
Embodiment 3
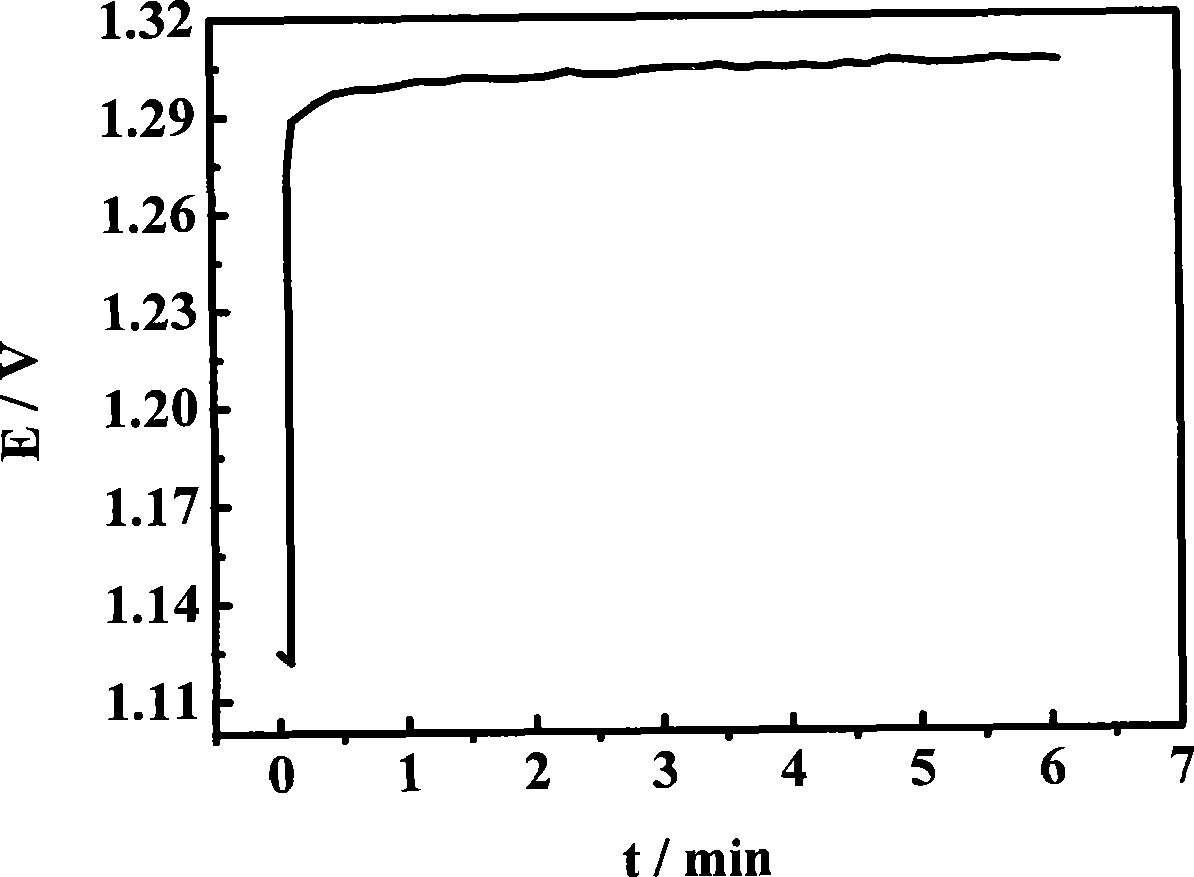
[0026] Mix 6 mL of ethanol and 4 mL of isopropanol to make 10 mL of organic solvent, then add 20 mg of H to the organic solvent 2 IrCl 6 and 3 mg H 2 PtCl 6 , ultrasonically disperse the mixed solution for 10 minutes to make a mixed solution; pipette 100 μL of the mixed solution per square centimeter of substrate area, and drop it on the surface of the titanium plate. After the organic solvent evaporates, put the titanium plate into a 60°C vacuum oven in vacuum drying for 15 minutes; put it into a tube furnace and sinter at 480°C for 5 minutes to make H 2 IrCl 6 、H 2 PtCl 6 Thermal decomposition to form metal and metal oxide coatings; take out the air and cool to room temperature, repeat the above steps until the required loading capacity is 10mg / cm 2 ;Finally placed in a tube furnace and sintered at 480°C for 1.5 hours, the prepared IrO 2 / Pt catalyst, performance such as image 3 shown.
[0027] By the result of the galvanostatic electrolysis test carried out to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com