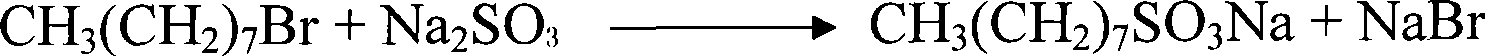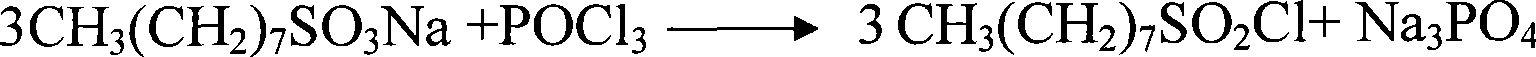Full-fluorine octyl sulfuryl fluoride production technology
A technology of perfluorooctanesulfonyl fluoride and fluorooctanesulfonyl fluoride, which is applied in the field of perfluorooctanesulfonyl sulfonyl fluoride production technology, can solve the problem that there is no public report on the chemical synthesis production method and process of perfluorooctanesulfonyl fluoride, The continuous electrolysis time and life of the plate are shortened, and the equipment utilization rate is low, and the continuous electrolysis time is extended, the equipment utilization rate is improved, and the production efficiency is improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
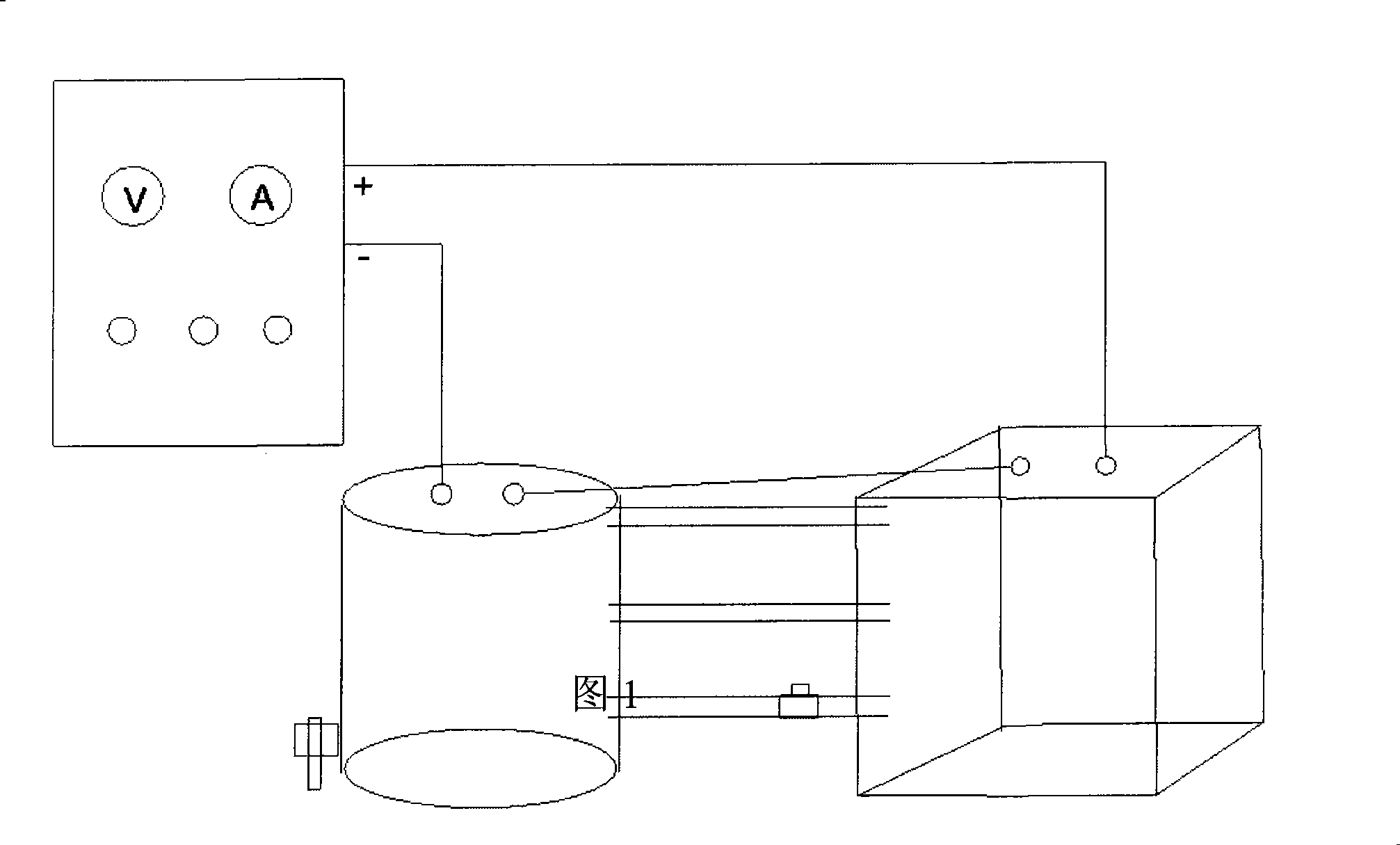
Image
Examples
Embodiment
[0021] The present invention uses bromooctane as a raw material, first undergoes sulfonation, and the reaction equation is as follows:
[0022]
[0023] Use alcohol as a solvent, control the reaction temperature between 80°C and 90°C, and react for 18 hours. When there is no oil flower-like liquid in the reaction tube, the reaction ends. The alcohol in the reaction residue is recovered and reused.
[0024] The product obtained by sulfonation is fully dried and sent to the chlorination section for chlorination.
[0025] The chlorination kettle needs to be cleaned and fully dried. Weigh the octyl sulfonate which is the reaction product of the first step, put phosphorus oxychloride into the reactor, start stirring, then add the weighed sulfonate into the reactor. The reaction equation for this step is:
[0026]
[0027] Keep the temperature of the kettle at 70° C. to 75° C. for 6 hours, and the reaction is basically completed. Wait until the temperature in the kettle dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com