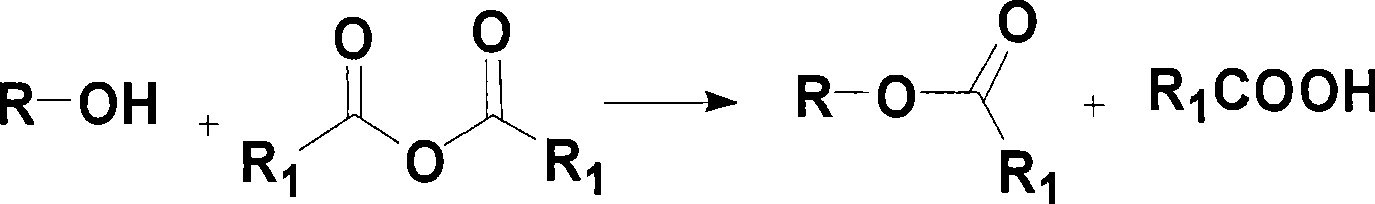
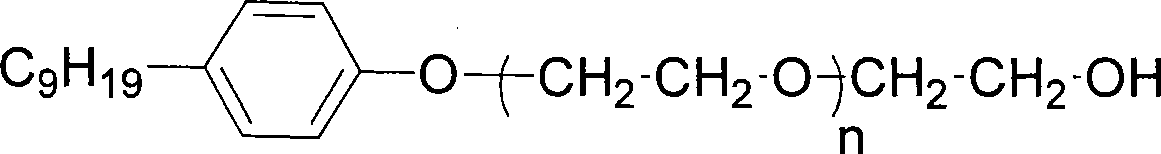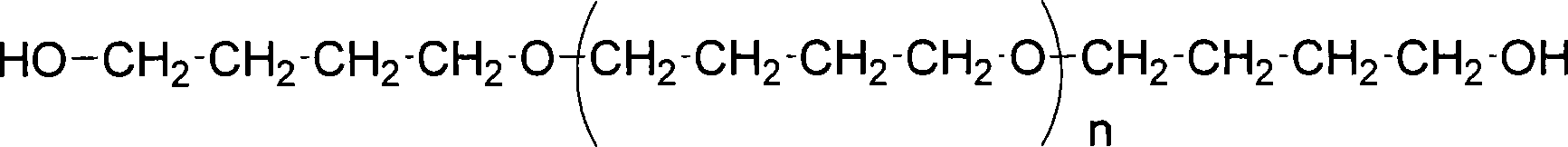Esterification reagent used for measuring hydroxyl value by estolide method, and method for measuring hydroxyl value
A technology of acid anhydride and hydroxyl value, which is applied in the direction of chemical analysis by titration method, analysis by making materials undergo chemical reactions, and material analysis by observing the influence on chemical indicators, etc., which can solve the problems of inconvenient operation of analysis tests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of Esterification Reagent for Measuring Polyol Hydroxyl Value
[0030] Esterification reagents are prepared according to known methods excluding atmospheric humidity. Use reagent grade chemicals.
[0031] Esterification reagent A: at room temperature, 2.0 g of MoO 2 (acac) 2 (6 mmol) was dissolved in 50 g of acetic anhydride (490 mmol) with magnetic stirring to prepare a solution. Afterwards, the above solution was transferred to a 100 ml measuring bottle, and diluted to the marked line with toluene under sufficient shaking. Store this esterification reagent A in a brown bottle.
[0032] Esterification reagent B: at room temperature, 1.3 grams of VO(OAc) 2 (6.6 mmol) was dissolved in 64 g of propionic anhydride (492 mmol) with magnetic stirring to prepare a solution. Afterwards, the above solution was transferred to a 100 ml measuring bottle, and diluted to the marked line with xylene under sufficient shaking. Store this esterification reagent B in a ...
Embodiment 2
[0045] Measure the hydroxyl value of commercial nonionic surfactant polyoxyethylenenonylphenyl ether (theoretical hydroxyl value is 75)
[0046]
[0047] n=12
[0048] First, 2.0115 grams and 2.022 grams of samples were measured and placed in flask 1 and flask 2, respectively. Then, each 5 milliliters of esterification reagent A was dripped into each sample to be tested and the flask used as blank measurement. The titration result of flask 1 is that 37.9 ml of 0.5N sodium hydroxide standard titration solution is consumed, and flask 2 also consumes 37.9 ml. The titration result of the blank test flask 1 is 43.3 milliliters of 0.5 N sodium hydroxide standard titration solution consumed, and the blank test flask 2 consumes 43.5 milliliters, so the average consumption of the blank test is 43.4 milliliters. The concentration (mol / L) of sodium hydroxide standard titration solution is 0.5015. According to the above formula, the hydroxyl value of the sample in flask 1 is calcula...
Embodiment 3
[0050] Measure the hydroxyl value of commercial poly(tetramethylene glycol) (marked hydroxyl value 107.8)
[0051]
[0052] n=14
[0053] First, 1.5514 grams and 1.5453 grams of samples were measured and placed in flask 1 and flask 2, respectively. Then, 5 milliliters of the esterification reagent B was dripped into each sample to be tested and the flask used as a blank measurement. The titration result of flask 1 was 38.1 ml of 0.5N sodium hydroxide standard titration solution consumed, and 38.3 ml of flask 2 was consumed. The titration result of the blank test flask 1 is the consumption of 44.2 milliliters of 0.5N sodium hydroxide standard titration solution, and the blank test flask 2 also consumes 44.2 milliliters, so the average consumption of the blank test is 44.2 milliliters. The concentration (mol / L) of sodium hydroxide standard titration solution is 0.4895. According to the above formula, the hydroxyl value of the sample in flask 1 is calculated to be 108.0, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com