Polyenic taxusol nano lipid carrier and preparation method thereof
A nano-lipid carrier and docetaxel technology, which is applied in the directions of liposome delivery, pharmaceutical formulations, and inactive medical preparations, can solve problems such as limiting drug-carrying capacity, improve injection safety, and avoid phagocytosis. , the effect of prolonging the cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
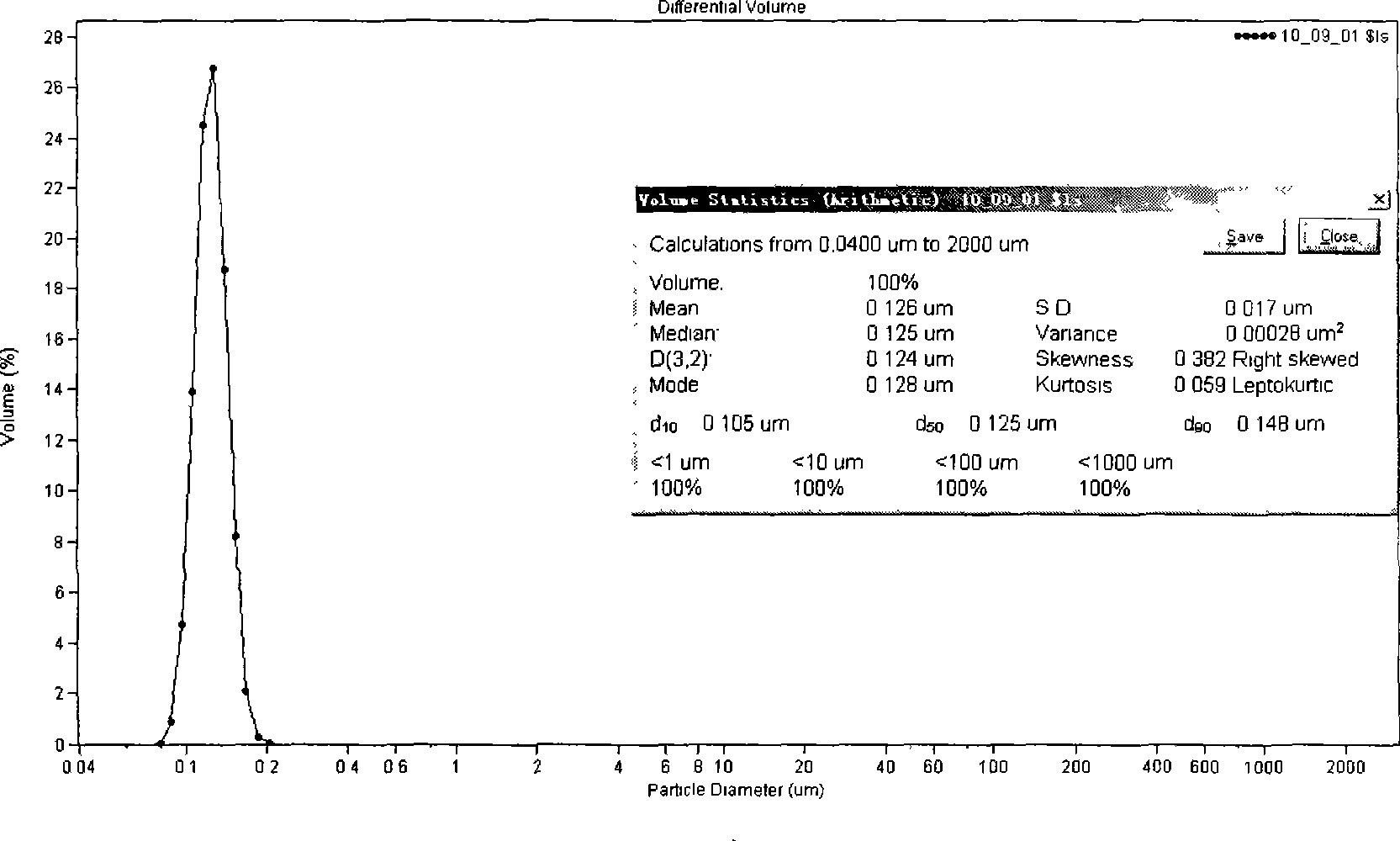
[0040] Take 600 mg of docetaxel, 10.0 g of glyceryl monostearate, 8.0 g of MCT, and 2.0 g of Tween80, add an appropriate amount of absolute ethanol to dissolve, and rotate the organic solvent under reduced pressure to dry the organic solvent, and heat and dissolve at 70°C to form the oil phase. Poloxamer 188 4.0g, dissolved in 100ml water for injection, heated to 70°C as the water phase. The water phase was dropped into the oil phase under stirring at 70°C, and the obtained colostrum was processed by a high-pressure homogenization method at 70°C, and the docetaxel nanolipid carrier was sealed and stored at 4°C. After diluting with physiological saline, measure the flat diameter particle size with COULTER LS230 particle size analyzer, see figure 1 .
[0041] Average particle size=126nm, SD=17nm, encapsulation rate=90.3wt%.
Embodiment 2
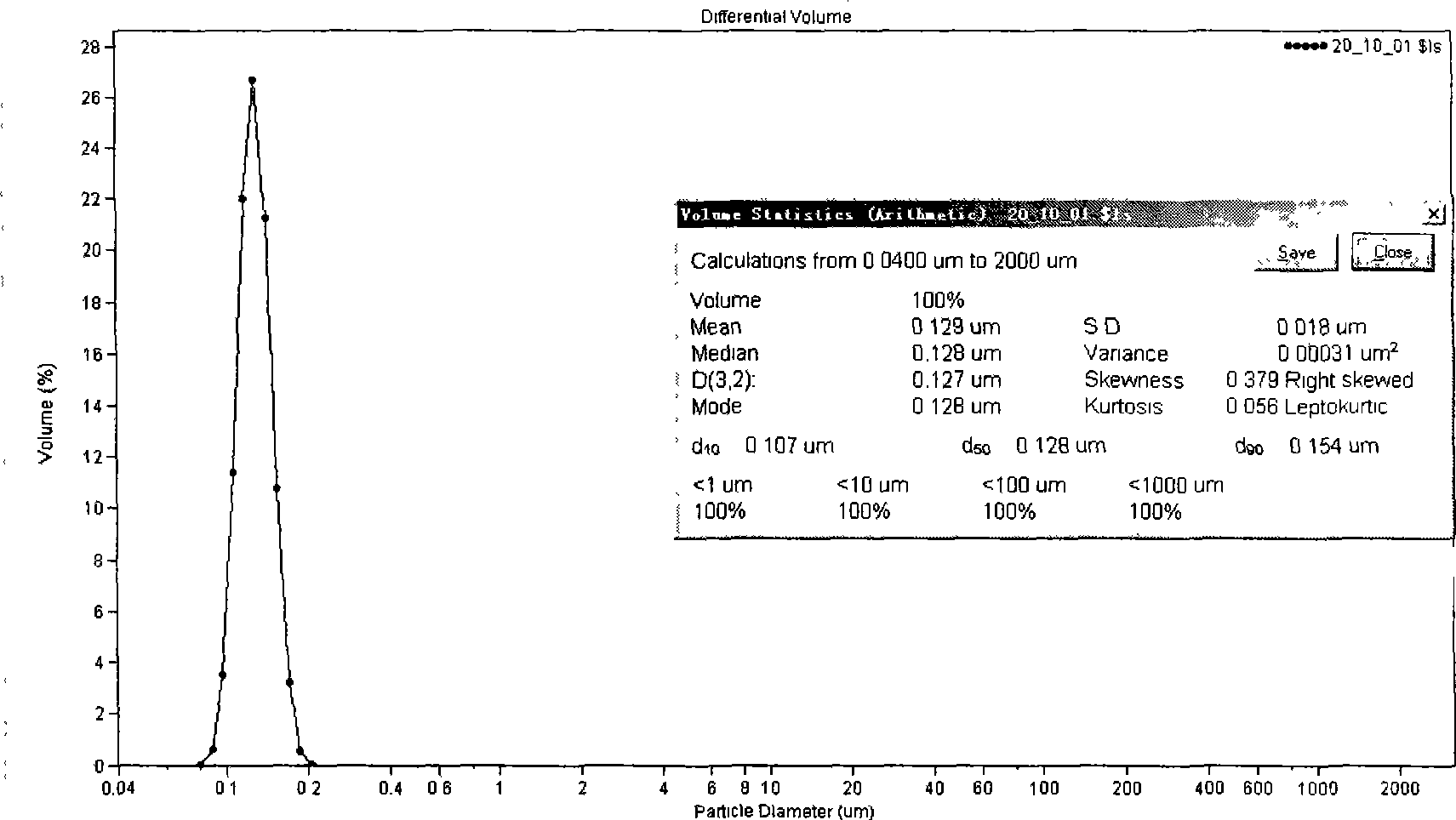
[0043] Take 60 mg of docetaxel, 1.0 g of glyceryl monostearate, 0.8 g of MCT, and 0.4 g of phospholipid to dissolve in an appropriate amount of chloroform, rotate and evaporate the organic solvent, and heat and dissolve at 70° C. as the oil phase. Poloxamer 188 0.3g is dissolved in 10ml water for injection and heated to 70°C as the water phase. The water phase was dropped into the oil phase under stirring at 70°C, the obtained colostrum was sonicated with a 500W probe at 70°C for 2 minutes, and the docetaxel nanolipid carrier was sealed and stored at 4°C. After diluting with physiological saline, measure the flat diameter particle size with COULTER LS230 particle size analyzer, see figure 2 .
[0044] Average particle size=129nm, SD=18nm, encapsulation rate=87.5wt%.
Embodiment 3
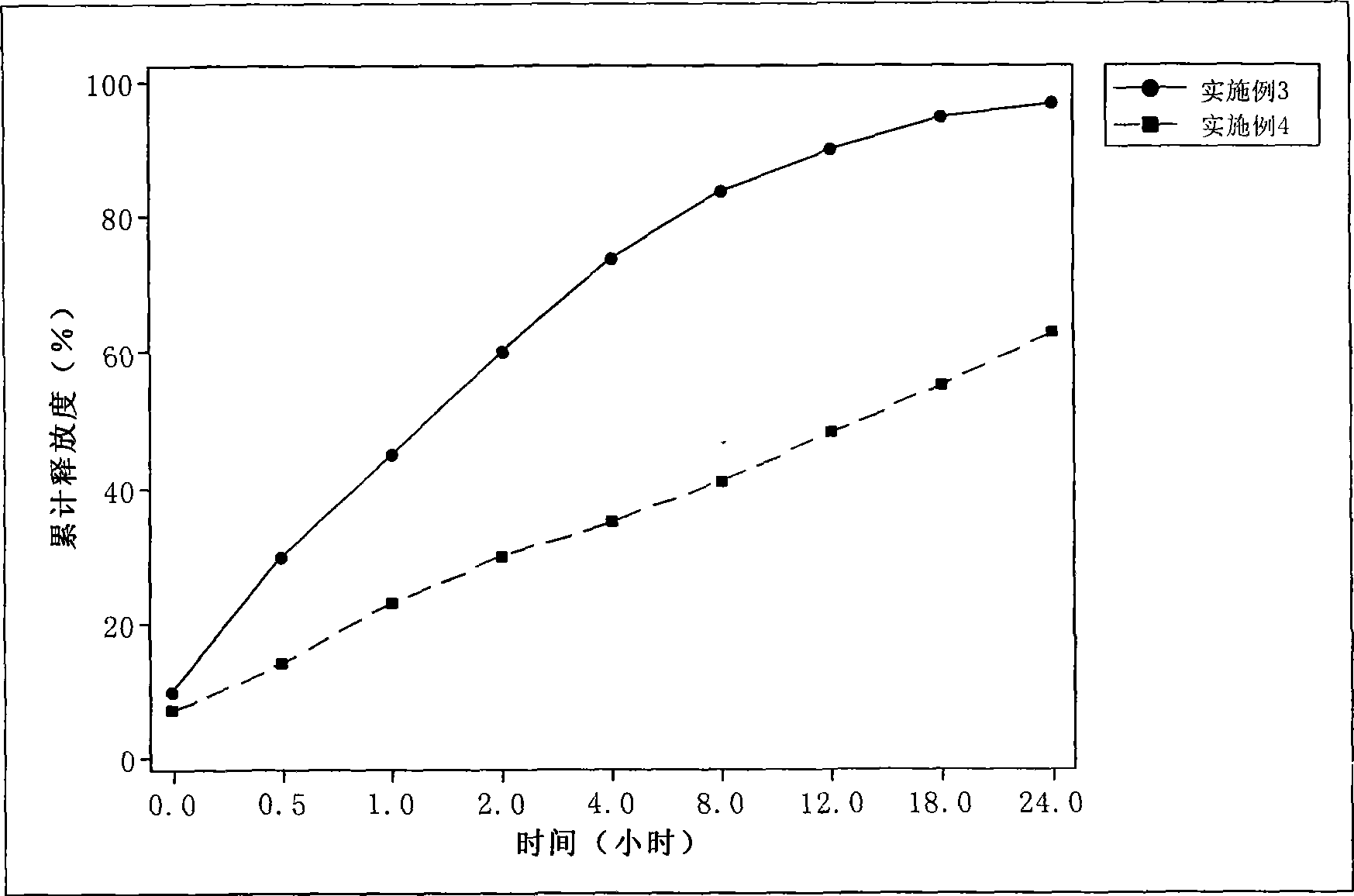
[0046] Take 500 mg of docetaxel, 8.0 g of glyceryl behenate, 5.0 g of soybean oil, and 1.5 g of Tween80, add an appropriate amount of chloroform to dissolve, dry the organic solvent under reduced pressure, and heat and dissolve at 80° C. as an oil phase. Dissolve 5.0 g of PEG 400 and 2.0 g of Poloxamer in 100 ml of water for injection, and heat to 80°C as the water phase. The water phase was dropped into the oil phase under stirring at 80°C, and the obtained colostrum was homogenized under high pressure at 70°C, and the docetaxel nanolipid carrier was sealed and stored at 4°C. The dialysis method was used to determine the drug release rate: 1000ml of phosphate buffered saline (PBS) containing 1% Tween and pH 7.4 was used as the release medium, the stirring speed was 100 revolutions per minute, and the temperature was 37±0.5°C. After sampling, inject 20μl for HPLC determination to calculate the cumulative release percentage, see image 3 .
[0047] Average particle size=133nm, SD=2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com