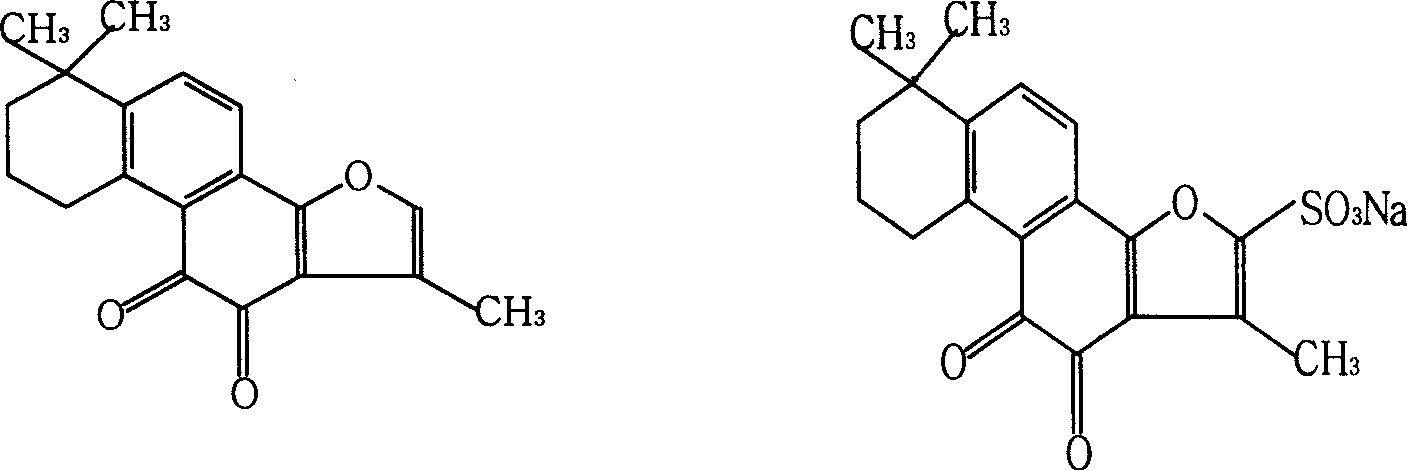
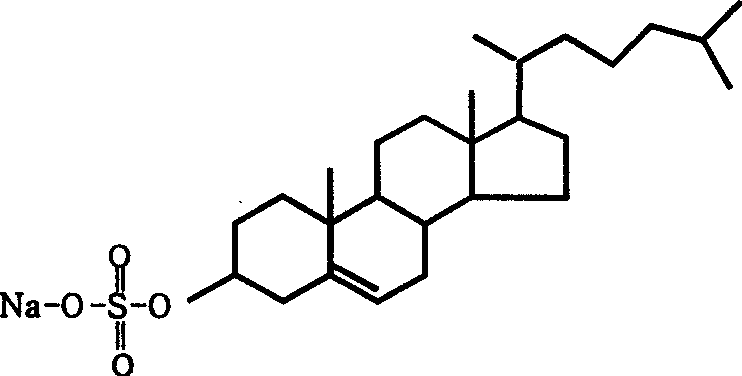
Tanshinone IIA lipid complexes and micelle composition thereof for injection
A lipoplex and tanshinone technology, applied in the direction of drug combination, drug delivery, medical preparations of non-active ingredients, etc., can solve the problem of adding steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Take 500mg of Tanshinone IIA, 2.0g of egg yolk lecithin, 400mg of sodium cholesterol sulfate, and 10mg of vitamin E, and dissolve them in methanol at 30°C as the organic phase; dissolve 0.5g of PEG400 in 90ml of water for injection, heat to 30°C as the water phase, The organic phase was injected into the water phase under stirring at 30°C, and the stirring was continued for 5 minutes, and then the methanol was evaporated under reduced pressure to obtain the tanshinone IIA lipid complex with blue opalescence, and its average particle size was measured to be 82±17nm. Add water for injection to make up to a total volume of 100ml, filter and sterilize with a 0.22μm microporous membrane, dispense 2ml / tube, and sterilize at 121°C for 30min to obtain an injection.
[0033] Add trehalose 5g to the prepared lipoplex, water for injection to 100ml, filter and sterilize with a 0.22μm microporous membrane, pack 2ml / branch, and freeze-dry under aseptic operation to obtain final product...
Embodiment 2
[0035] Take 1.0g of tanshinone IIA, 3.0g of soybean lecithin, and 500mg of sodium cholesterol sulfate, and dissolve them in absolute ethanol at 35°C as the organic phase; dissolve 1.0g of PEG200 in 90ml of water for injection, heat to 35°C as the water phase, and The organic phase was injected into the water phase under stirring at 35°C, and the stirring was continued for 10 minutes, and then the ethanol was evaporated under reduced pressure to obtain the tanshinone IIA lipid complex with blue opalescence, and its average particle size was measured to be 72±12nm. Add water for injection to make up to a total volume of 100ml, filter and sterilize with a 0.22μm microporous membrane, dispense 2ml / tube, and sterilize at 121°C for 30min to obtain an injection.
[0036] Add trehalose 5g to the prepared lipoplex, water for injection to 100ml, filter and sterilize with a 0.22μm microporous membrane, pack 4ml / branch, and freeze-dry under aseptic operation to obtain final product. The f...
Embodiment 3
[0038] Take 2.0 mg of tanshinone IIA, 10 g of soybean lecithin, and 5 g of sodium cholesterol sulfonate, and dissolve it in tetrahydrofuran at 35 °C as the organic phase; dissolve 1885 g of poloxamer in 950 ml of water for injection, heat it to 35 °C as the water phase, and stir at 35 °C Inject the organic phase into the water phase, continue to stir for 10 minutes, and then evaporate the tetrahydrofuran under reduced pressure to obtain the tanshinone IIA lipid complex with blue opalescence. For 100ml, use a 0.22μm microporous membrane to filter and sterilize, aliquot 2ml / tube, and sterilize at 121°C for 30min to obtain the injection.
[0039] Add trehalose 5g to the prepared lipoplex, water for injection to 100ml, filter and sterilize with a 0.22μm microporous membrane, pack 4ml / branch, and freeze-dry under aseptic operation to obtain final product. The freeze-dried preparation was diluted with physiological saline, and the average particle size was measured to be 70±18nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com