Paclitaxel nano lipid carrier and preparation method thereof
A nano-lipid carrier, paclitaxel technology, applied in the directions of liposome delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc. Narrow distribution of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
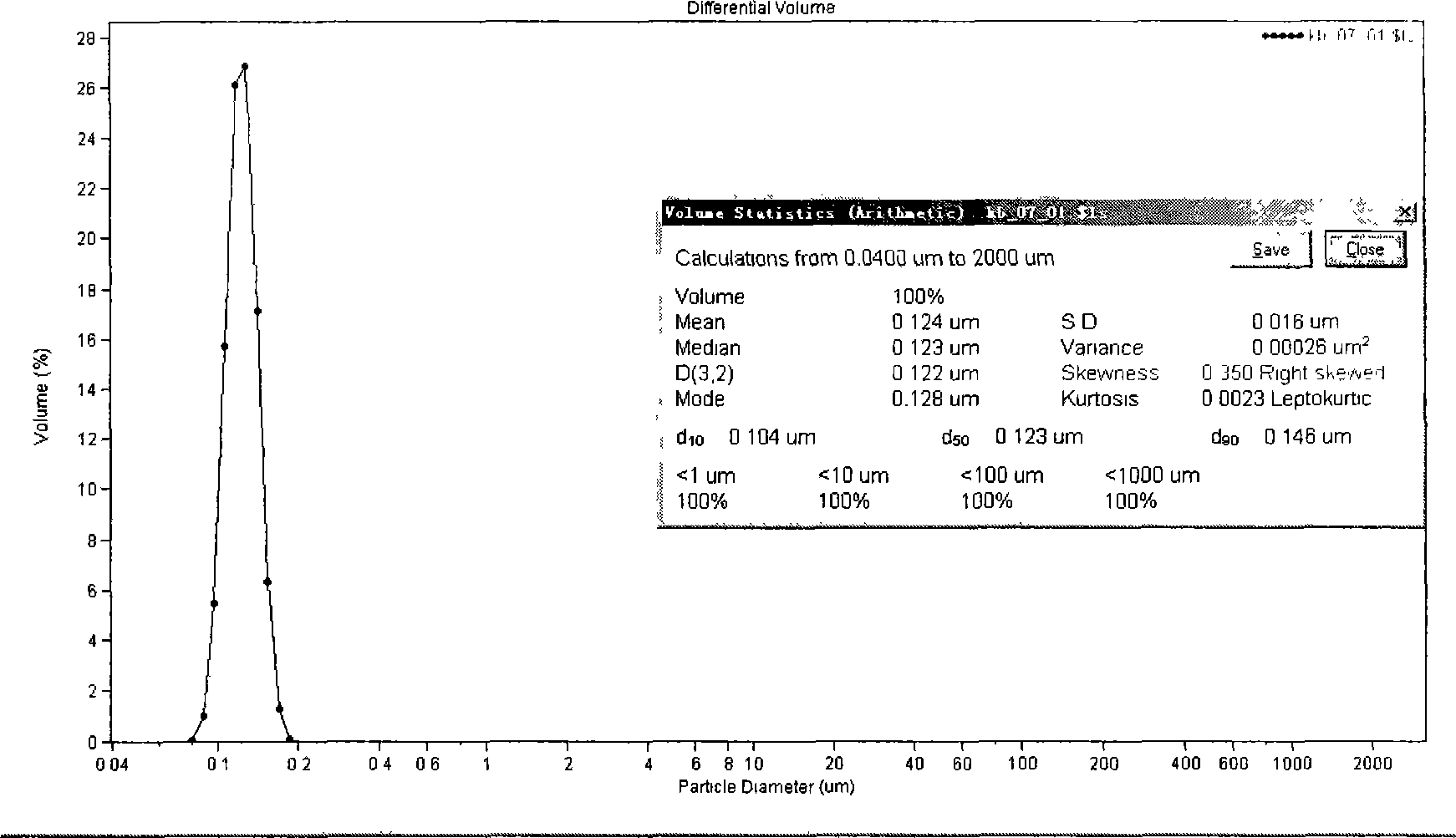
[0037] Take 800mg of paclitaxel, 12.0g of glyceryl monostearate, 10.0g of MCT, 803.0g of Tween, add appropriate amount of ethanol to dissolve, evaporate the organic solvent under reduced pressure, and heat to dissolve at 70°C as the oil phase. Poloxamer 188 3.0g, dissolved in 100ml of water for injection, heated to 70°C as the water phase. The water phase was dropped into the oil phase under stirring at 70°C, and the obtained colostrum was homogenized under high pressure at 70°C to obtain paclitaxel nano-lipid carrier, which was sealed and stored at 4°C. After diluting with normal saline, measure the average diameter particle size with a COULTER LS230 particle size analyzer, see figure 1 .
[0038] Average particle diameter = 124nm, SD = 16nm, encapsulation efficiency = 87.9wt%.
Embodiment 2
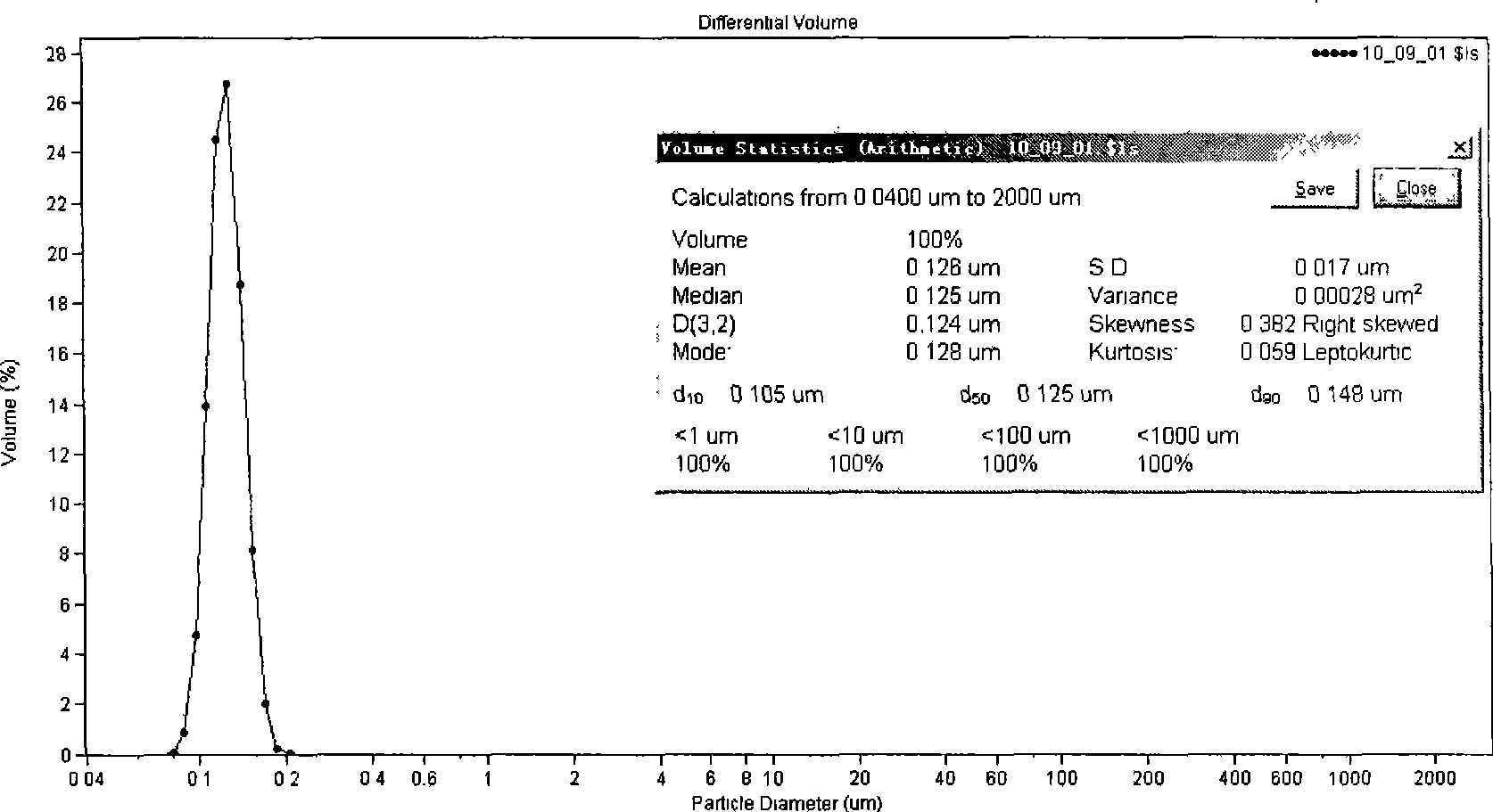
[0040] Take 80 mg of paclitaxel, 1.2 g of glyceryl monostearate, 1.1 g of MCT, and 1.2 g of phospholipids, add an appropriate amount of chloroform to dissolve, spin evaporate the organic solvent to dryness, heat and dissolve at 70°C as the oil phase. Poloxamer 1880.6g was dissolved in 10ml water for injection and heated to 70°C as the aqueous phase. The water phase was dropped into the oil phase under stirring at 70°C, and the obtained colostrum was sonicated with a 400W probe at 70°C for 3 minutes to obtain paclitaxel nano-lipid carrier, which was sealed and stored at 4°C. After diluting with normal saline, measure the average diameter particle size with a COULTER LS230 particle size analyzer, see figure 2 .
[0041] Average particle diameter = 126nm, SD = 17nm, encapsulation efficiency = 88.4wt%.
Embodiment 3
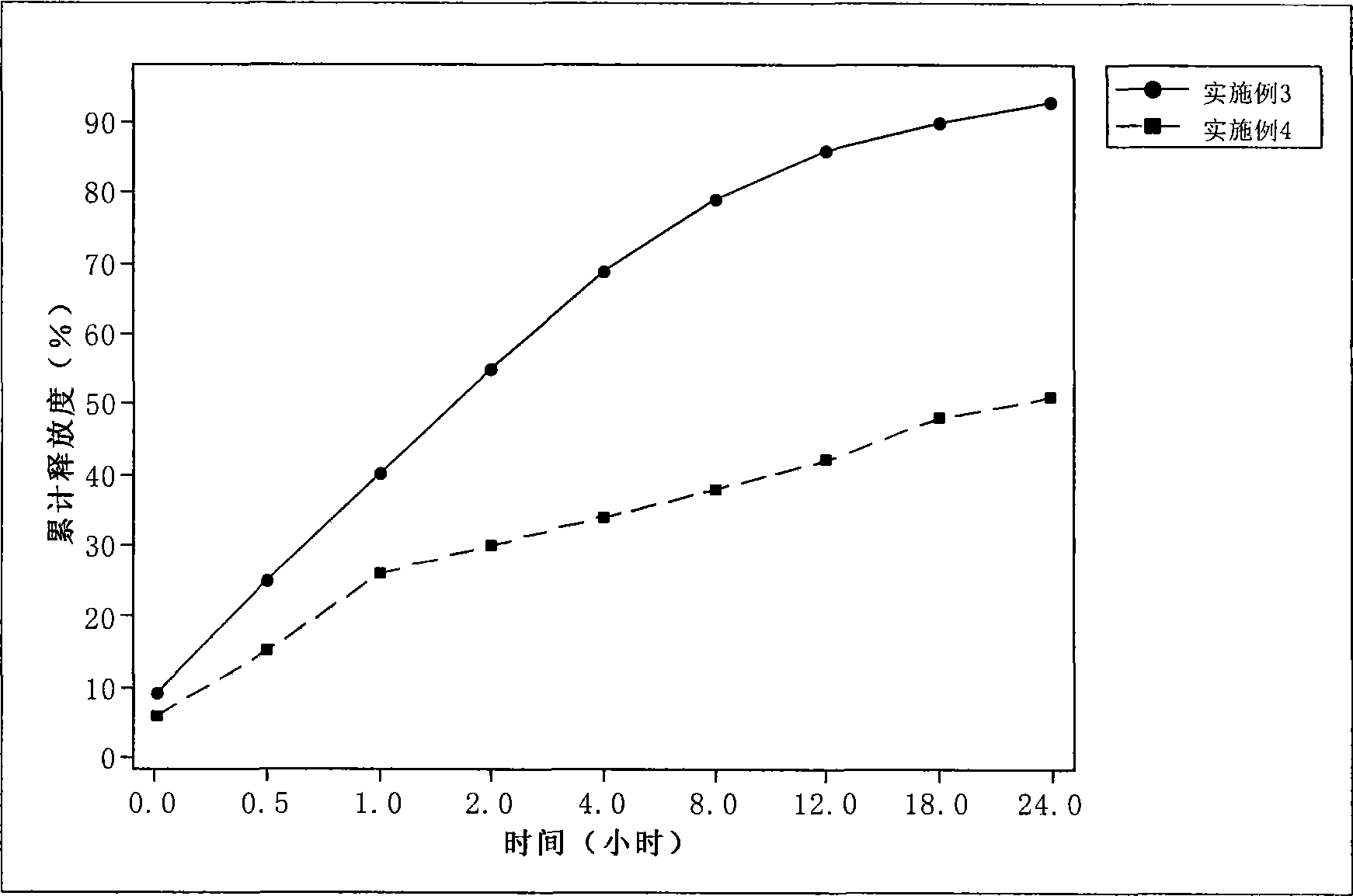
[0043] Take 800 mg of paclitaxel, 12.0 g of glyceryl behenate, 7.0 g of soybean oil, and 803.5 g of Tween, add appropriate amount of tetrahydrofuran to dissolve, rotary evaporate the organic solvent to dryness, heat and dissolve at 80°C as the oil phase. Dissolve 3.0 g of Poloxamer 1888 in 100 ml of water for injection, and heat to 80° C. as the water phase. The water phase was dropped into the oil phase under stirring at 70°C, and the obtained colostrum was homogenized under high pressure at 70°C to obtain paclitaxel nano-lipid carrier, which was sealed and stored at 4°C. Adopt dialysis to measure the release degree of medicine: take the phosphate buffer saline (PBS) 1000ml that contains 1% Tween as pH value 7.4 as release medium, stirring speed is 100 revolutions per minute, and temperature is 37 ± 0.5 ℃. After sampling, inject 20 μl of sample for HPLC determination, and calculate the cumulative drug release percentage, see image 3 .
[0044] Average particle diameter = 13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com