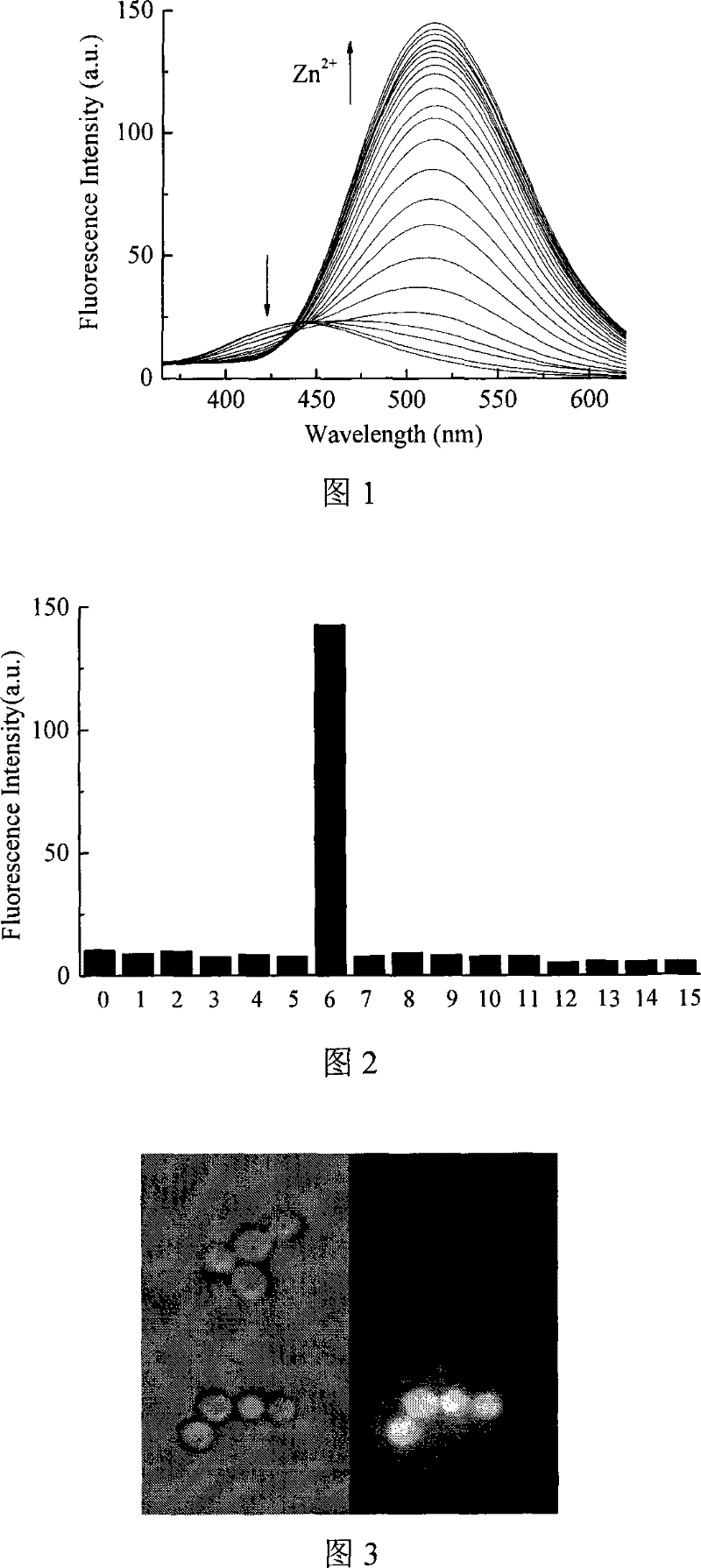
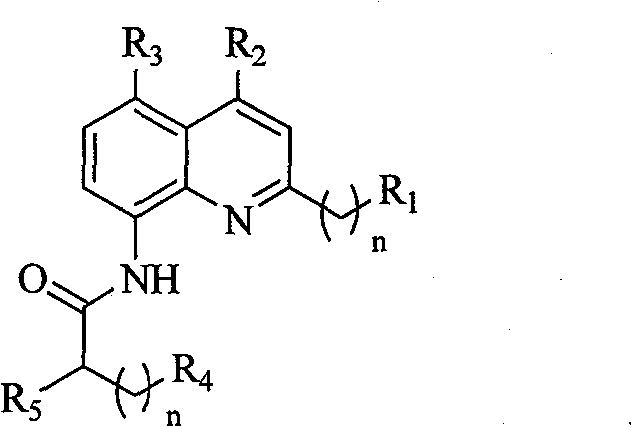
Synthesis of N-acyl-8-amino quinoline derivatives and use thereof as fluorescent molecular probe
An aminoquinoline and hydrocarbylamino technology, which is used in the synthesis of N-acyl-8-aminoquinoline derivatives and its application as a fluorescent molecular probe, can solve the problems of short emission wavelength, poor water solubility, weak fluorescence and the like , to achieve the effect of enhanced fluorescence intensity, easy availability of raw materials and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Dissolve 288mg (2.0mmol) of 8-aminoquinoline and 222mg (2.8mmol) of pyridine in 10mL of chloroform, add 5mL of chloroform solution containing 461mg (2.4mmol) of chloroacetyl chloride dropwise within 1 hour under ice cooling, Stir at room temperature for 2h to stop the reaction. The reaction liquid was spin-dried and purified by column chromatography, the yield was 79.9%, mp: 131.6-132.6°C. Further 80mg (0.362mmol) 8-chloroacetyl quinoline, 381mg (3.62mmol) aminoethoxyethanol, 468mg (3.62mmol) DIPEA and 20mgKI were added to 30mL acetonitrile, N 2 Under the protection of reflux for 10h, stop the reaction. The reaction solution was spin-dried and purified by column chromatography with a yield of 83.8%. 1 H-NMR (400MHz, CDCl 3 ): 11.24(CONH, s, 1H), 8.86(d, J=5.6Hz, 1H), 8.81(d, J=8.8Hz, 1H), 8.16(d, J=9.6Hz, 1H), 7.53(m , 2H), 7.46(m, 1H), 3.74(OCH 2 CH 2 -OH, m, 4H), 3.59 (COCH 2 , NHCH 2 -CH 2 , m, 4H), 2.99 (CH 2 NH-CH 2 , t, J=4.8Hz, 2H).
Embodiment 2
[0029]
[0030] Operation method is referring to embodiment 1. Yield: 79.4%, mp: 112.4°C. 1H-NMR (400MHz, DMSO): 8.95(d, J=8.0Hz, 1H), 8.69(d, J=8.8Hz, 1H), 8.43(d, J=9.6Hz, 1H), 7.66(m, 3H ), 3.67 (CH 2 -OH, t, J=5.2Hz, 2H), 3.48 (COCH 2 , s, 2H), 3.14 (NH-CH 2 CH 2 OH, t, J=6.4Hz, 2H), 3.09 (NHCH 2 -CH 2 NH, t, J=5.2Hz, 2H), 2.92 (COCH 2 NH-CH 2 , t, J=6.6Hz, 2H).
Embodiment 3
[0032]
[0033] Operation method is referring to embodiment 1. Yield: 62.7%, mp: 183.0-184.2°C. 1 H-NMR (400MHz, DMSO): 8.97 (d, J = 5.2Hz, 1H), 8.63 (d, J = 7.6Hz, 1H), 8.45 (d, J = 9.6Hz, 1H), 8.76 (d, J =8.0Hz, 2H), 7.66(m, 2H), 4.12(COCH 2 , s, 2H), 3.69 (CH 2 -OH, t, J=5.4Hz, 2H), 3.05(CH 2 NH-CH 2 , t, J=5.4Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com