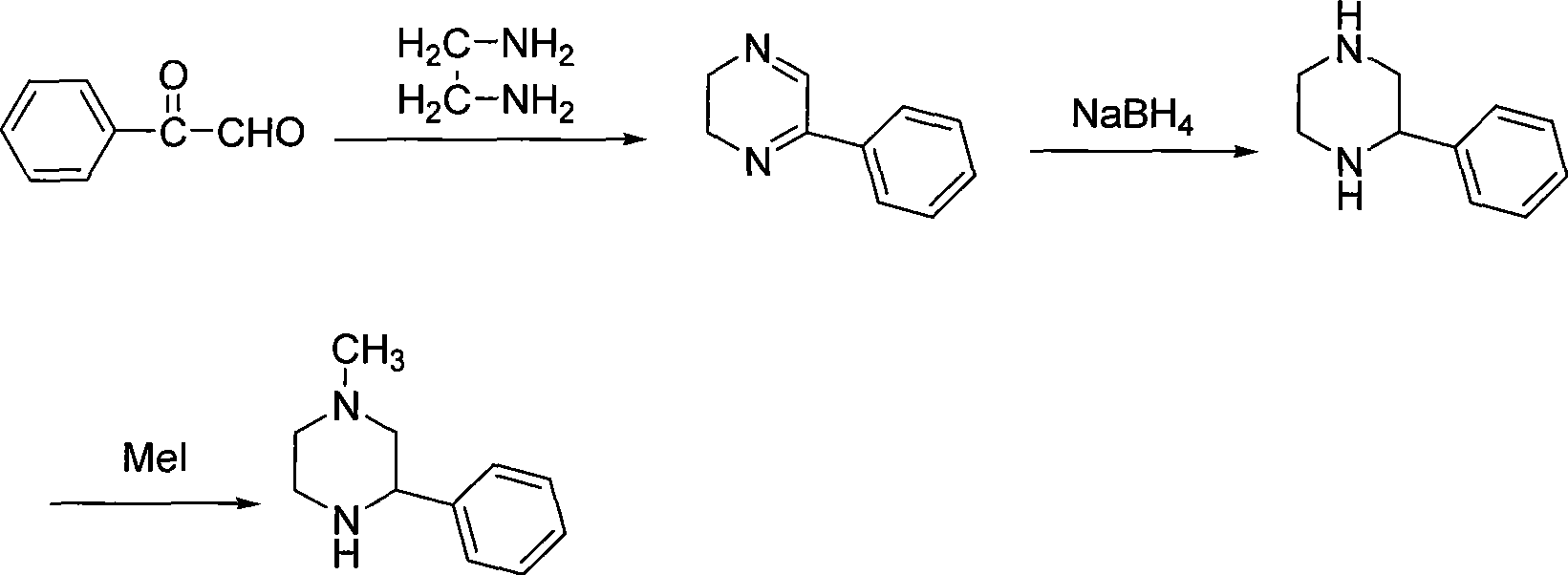
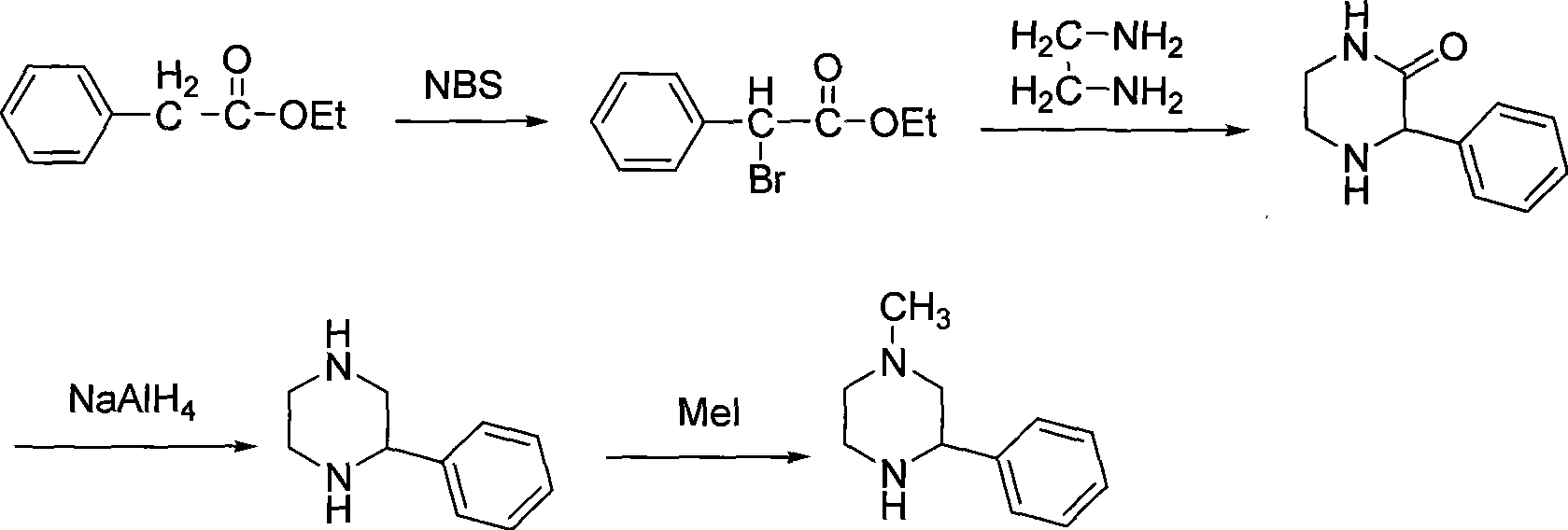
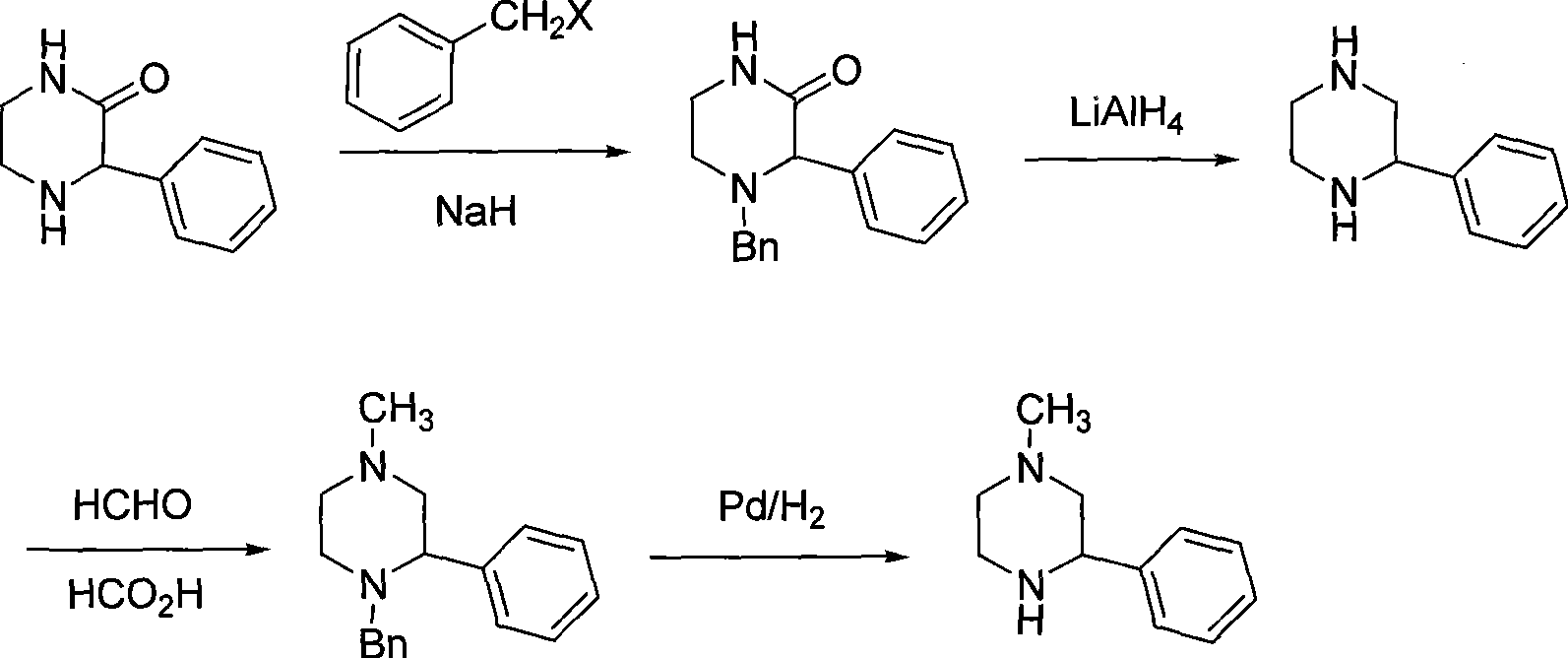
Preparation of medicament intermediate 1-methyl-3-phenyl piperazine
A technology for phenylpiperazine and intermediates, which is applied in the field of preparation of pharmaceutical intermediates 1-methyl-3-phenylpiperazine, can solve the problems of low yield, achieve high reaction yield, simple post-treatment, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] A preparation method of a pharmaceutical intermediate MPP, the preparation method comprising the following steps:
[0076] (1) Preparation of compound II:
[0077] Step 1: Add 75 grams of styrene oxide (0.6 mol) and 150 ml of toluene into a 500 ml three-necked flask equipped with mechanical stirring and a condenser, heat to 80±5°C, and add 43 grams of N-methylethanolamine dropwise (0.57mol), dripped in about 1.5 hours, then incubated at the same temperature for about 2 hours, determined the end point of the reaction with TLC, and cooled to room temperature for use;
[0078] Step 2: Add 300ml of toluene and 170 grams of thionyl chloride (1.42mol) to another 1000ml three-necked flask, cool to 0-10°C with an ice-water bath, and slowly drop the reaction mixture obtained in the first step under stirring Add it into the 1000ml three-neck flask, and finish adding in about 2 hours. Control the temperature at 0-10°C. After the dropwise addition, slowly heat the system to room t...
Embodiment 2
[0085] A preparation method of a pharmaceutical intermediate MPP, the preparation method comprising the following steps:
[0086] (1) Preparation of compound II:
[0087] Step 1: Put 130kg of toluene into a 500-liter reactor, start stirring, add 80kg of styrene oxide, raise the temperature to 80-85°C, add 45.5kg of N-methylethanolamine dropwise, and finish dropping in about 80-90 minutes , heat preservation reaction, use TLC to spot the plate until the raw material reaction is complete, react for about 3 hours, cool to room temperature, and move to the high tank of the next step reaction;
[0088] The second step: put 86kg of toluene in a 1000 liter reaction kettle, slowly add 162kg of thionyl chloride (note the exotherm), cool the system down to 0-5°C, start adding the first step reaction solution dropwise, and keep the temperature at 0~10°C, drop it in about 3 hours, raise the temperature to room temperature (20±5°C) and stir for 30 minutes, then raise the temperature to 45...
Embodiment 3
[0097] A preparation method of a pharmaceutical intermediate MPP, the preparation method comprising the following steps:
[0098] (1) Preparation of compound II:
[0099] The first step: In a 500ml three-necked flask equipped with mechanical stirring and a condenser, add 71.25 grams of styrene oxide (0.57mol) and 150ml of toluene, heat to 40°C, and add dropwise 43 grams of N-methylethanolamine (0.57mol) mol), the dropwise addition was completed, and the reaction was then incubated at the same temperature for about 2 hours, and the end point of the reaction was determined by TLC, and the temperature was lowered to room temperature for use;
[0100] The second step: add 300ml of toluene and 136.5 grams of thionyl chloride (1.14mol) to another 1000ml three-necked flask, cool to 0-10°C with an ice-water bath, and slowly drop the reaction mixture obtained in the first step under stirring Add it into the 1000ml three-neck flask, control the temperature at 0-10°C, after the dropwise...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com