Sterilized nanoparticulate glucocorticosteroid formulations
A technology of glucocorticoids and granules, applied in nanostructure manufacturing, nanotechnology, nanotechnology, etc., can solve problems such as contaminated drugs, difficulties, and time-consuming processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0181] D. Preparation method of the composition of the present invention
[0182]In another aspect of the present invention, a method for preparing the nanoparticle glucocorticoid preparation of the present invention is provided. The method includes one of the following methods: grinding or milling (including but not limited to wet milling), homogenization, precipitation, freezing, template emulsion method, supercritical fluid method, nano electrospray method, or any combination thereof. Exemplary methods of preparing nanoparticle compositions are described in US Patent No. 5,145,684. Methods of preparing nanoparticle compositions are also described in U.S. Pat. No. 5,518,187 "Method of Grinding Pharmaceutical Substances", U.S. Pat. 5,862,999 "Method of Grinding Pharmaceutical Substances (method of grinding drugs)", US Patent No. 5,665,331 "Co-Microprecipitation of Nanoparticulate Pharmaceutical Agents with Crystal Growth Modifiers (co-microprecipitation of nanoparticle drugs...
Embodiment 1
[0250] The purpose of this example was to evaluate the particle size of budesonide nanoparticle dispersions with polysorbate 80 as non-ionic surface stabilizer in the presence and absence of the amphipathic lipid lecithin.
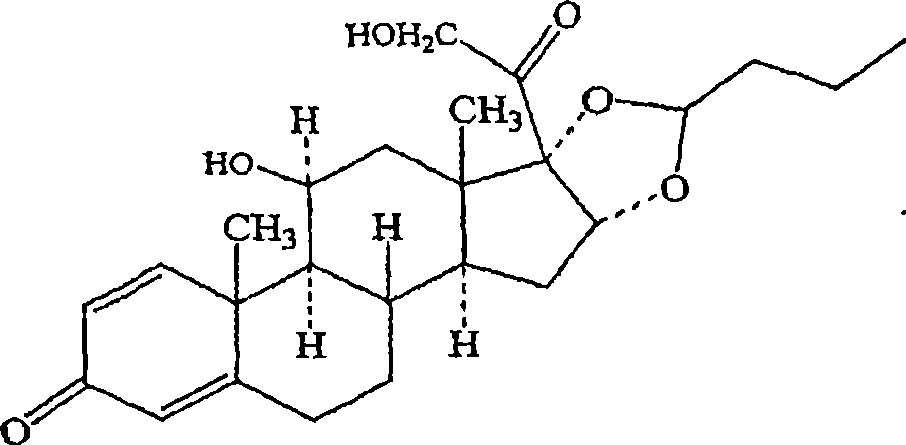
[0251] Budesonide has the following formula:
[0252]
[0253] Budesonide is known chemically as an acetal of (RS)-11,16,17,21-tetrahydroxy-pregna-1,4-diene-3,20-dionecyclo16,17-diol and butyraldehyde . Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula for budesonide is C 25 h 34 o 6 , and its molecular weight is 430.5.
[0254] Budesonide is a white to off-white odorless powder, almost insoluble in water and heptane, slightly soluble in ethanol, and easily soluble in chloroform.
[0255] A drug containing 30% (w / w) budesonide and 1.5% (w / w) polysorbate was prepared by adding 10 g polysorbate-80 to 456.7 g sterile water for injection (Abbott Labs) and 200 g budesonide (Farmabios) Aqueous colloidal dispersion ...
Embodiment 2
[0266] The purpose of this example is to determine the effects of different amounts of nonionic surface stabilizers and amphiphilic lipids on the particle size of nanoparticle budesonide dispersions after high pressure heat treatment.
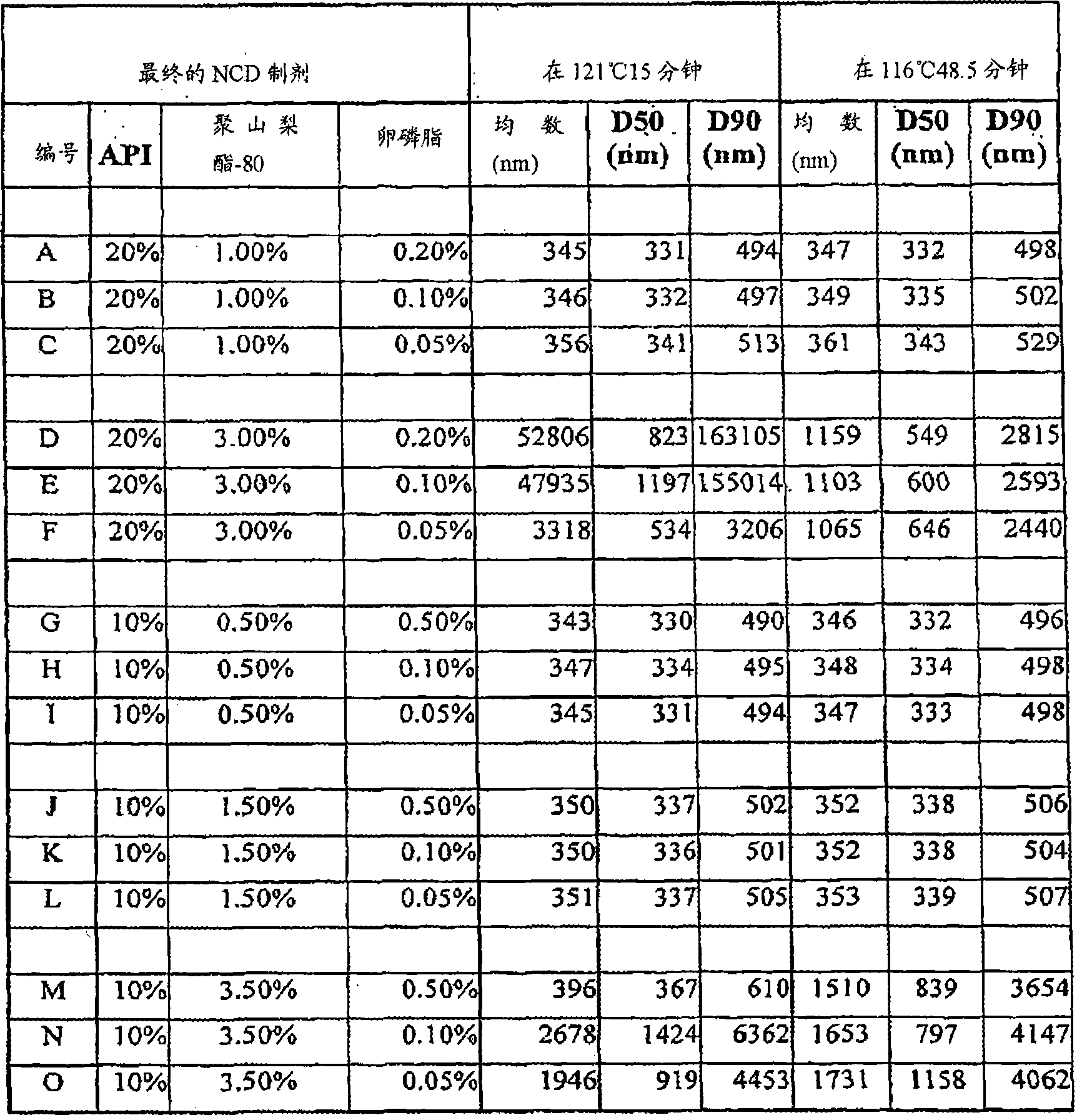
[0267] A separate portion of the 30% budesonide, 1.5% polysorbate-80 milled dispersion described in Example 1 was further diluted and various levels of sterile water for injection (SWFI), lecithin NF and polysorbate-80 were added. 80 was compounded to examine the effect of different percentages of polysorbate-80 and lecithin NF on the particle size of budesonide after autoclaving heat treatment. The effect of different hyperbaric exposure temperatures is also listed in Table II ("API" is active pharmaceutical ingredient or budesonide). All in Table II are percent by weight.
[0268] Table II: Particle size of budesonide dispersions after autoclave heat treatment: effect of different percentages of polysorbate-80 and lecithin NF
[0269]
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com